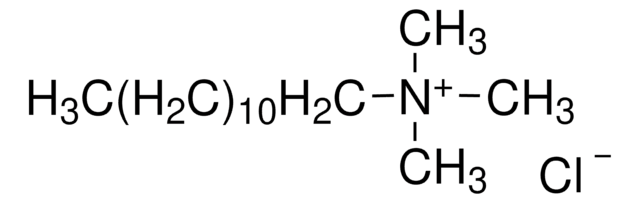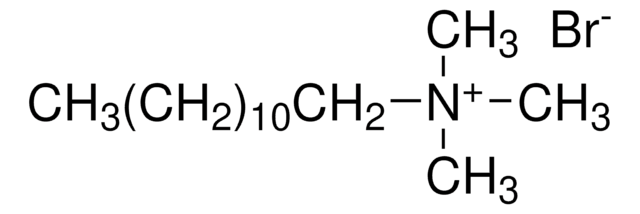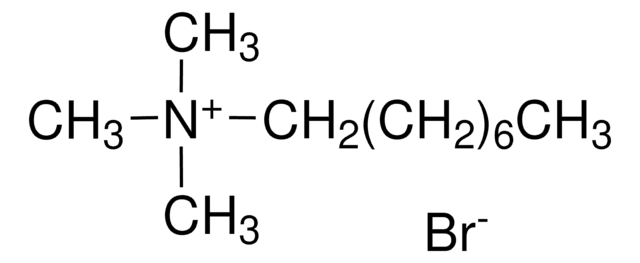44242
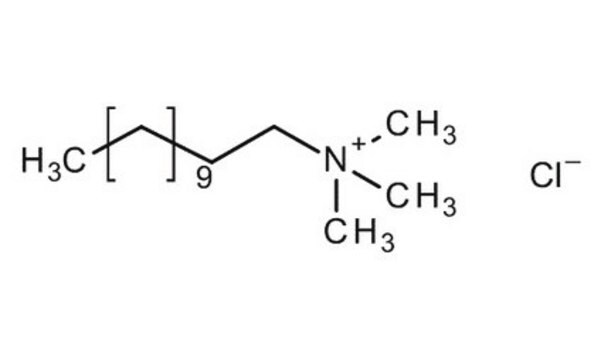
Dodecyltrimethylammonium chloride
≥99.0% (AT)
Synonym(s):
DTAC
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
CH3(CH2)11N(CH3)3Cl
CAS Number:
Molecular Weight:
263.89
Beilstein:
3915951
EC Number:
MDL number:
UNSPSC Code:
12352107
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
description
cationic
Assay
≥99.0% (AT)
form
solid
mol wt
263.89 g/mol
mp
37 °C
solubility
H2O: 50 mg/mL, clear, colorless
SMILES string
[Cl-].CCCCCCCCCCCC[N+](C)(C)C
InChI
1S/C15H34N.ClH/c1-5-6-7-8-9-10-11-12-13-14-15-16(2,3)4;/h5-15H2,1-4H3;1H/q+1;/p-1
InChI key
DDXLVDQZPFLQMZ-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
General description
Dodecyltrimethylammonium chloride is a cationic alkyltrimethylammonium chloride surfactant that can undergo micellization in aqueous solution. it is mainly used for the denaturation of proteins.
Application
Dodecyltrimethylammonium chloride has also been used as a cationic surfactant in:
- Transfer hydrogenation reactions of ketones/aldehydes.
- Ring-opening metathesis polymerization of olefins.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Phase separation in the isolation and purification of membrane proteins.
Arnold T
Biotechniques, 43(4), 427-442 (2007)
Temperature and salt-induced micellization of dodecyltrimethylammonium chloride in aqueous solution: A thermodynamic study.
Sarac B
Journal of Colloid and Interface Science, 338(1), 216-221 (2009)
Effect of temperature on critical micelle concentration and thermodynamic behavior of dodecyldimethylethylammonium bromide and dodecyltrimethylammonium chloride in aqueous media.
Mehta S
Colloids and Surfaces. A, Physicochemical and Engineering Aspects, 255(1-3), 153-157 (2005)
Jonas Carlstedt et al.
Physical chemistry chemical physics : PCCP, 14(27), 9574-9577 (2012-06-14)
Polyelectrolytes with amphiphilic counterions, PEACs, are water insoluble because the amphiphiles self-assemble into highly charged micelles that strongly associate with the equally highly charged polyions. However, in the presence of water soluble cyclodextrins (CDs) that form inclusion complexes with the
Marcos A Villetti et al.
The journal of physical chemistry. B, 115(19), 5868-5876 (2011-04-27)
Interactions between uncharged polymers and cationic surfactants are considered weaker than interactions with the anionic analogues. This work describes the binding occurring between methylcellulose (MC) and the cationic surfactant DTAB in aqueous medium. In the absence of salt, MC-DTAB exhibits
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service