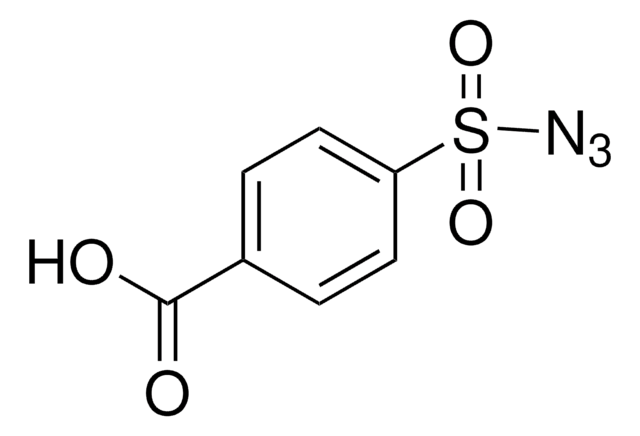
404764
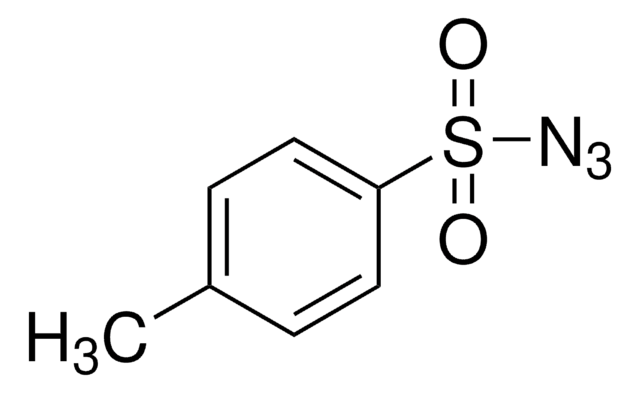
4-Acetamidobenzenesulfonyl azide
97%
Synonym(s):
p-ABSA
About This Item
Recommended Products
Quality Level
Assay
97%
form
solid
reaction suitability
reaction type: click chemistry
mp
107-111 °C (lit.)
SMILES string
CC(=O)Nc1ccc(cc1)S(=O)(=O)N=[N+]=[N-]
InChI
1S/C8H8N4O3S/c1-6(13)10-7-2-4-8(5-3-7)16(14,15)12-11-9/h2-5H,1H3,(H,10,13)
InChI key
NTMHWRHEGDRTPD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
Monosaccharide-derived alcohols
Non-peptidic NK3 receptor antagonists
Reagent for:
A late-stage intermolecular C-H olefination
Intramolecular isomuenchnone cycloaddition approach to antitumor agents
Rhodium-catalyzed carbene cyclization cycloaddition cascade reaction of vinylsulfonates
Suzuki-Miyaura cross coupling reaction
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
The chemistry of organoazides is exceedingly rich, since the azide functionality reacts with electrophiles, nucleophiles, and dipolarophiles, with or without the extrusion of dinitrogen. Common place transformation such as Staudinger reductions or ligations, Cu(I)-catalyzed Huisgen cycloadditions (of the “click” reaction family), Curtius or Schmidt rearrangents, nitrene reactions, or imine formation via aza-Wittig reactions all necessitate organoazide precursors or intermediates
Organic Azides and Azide Sources
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)

![1,4-Diazabicyclo[2.2.2]octane ReagentPlus®, ≥99%](/deepweb/assets/sigmaaldrich/product/structures/366/129/a6ff4175-974d-4fac-9038-b35e508ef252/640/a6ff4175-974d-4fac-9038-b35e508ef252.png)