402028
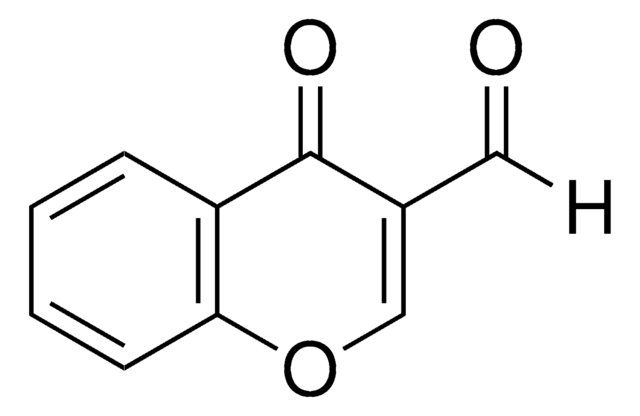
2-Amino-3-formylchromone
97%
Synonym(s):
2-Amino-4-oxo-4H-1-benzopyran-3-carboxaldehyde
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H7NO3
CAS Number:
Molecular Weight:
189.17
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
249 °C (dec.) (lit.)
functional group
aldehyde
ketone
SMILES string
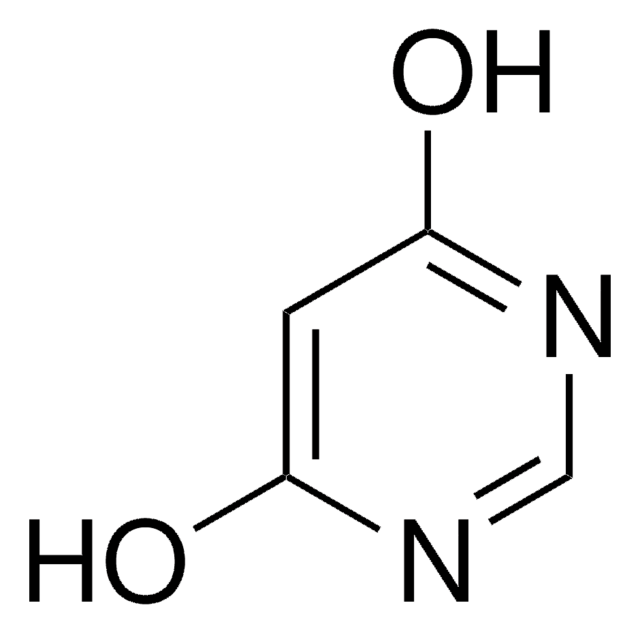
[H]C(=O)C1=C(N)Oc2ccccc2C1=O
InChI
1S/C10H7NO3/c11-10-7(5-12)9(13)6-3-1-2-4-8(6)14-10/h1-5H,11H2
InChI key
TVGIYZVZBKAJRR-UHFFFAOYSA-N
Gene Information
human ... PTPN1(5770)
General description
2-Amino-3-formylchromone (2-Amino-4-oxo-4H-1-benzopyran-3-carboxaldehyde) is a benzopyran derivative. It is a bicyclic heterocyclic molecule made up of a benzene ring fused to a heterocyclic pyran ring. It undergoes condensation with (R)-2-amino-2-phenylethanol to afford Schiff base ligand, which forms complexes with Cu(NO3)2 and Zn(NO3)2.
Application
2-Amino-3-formylchromone may be used in the preparation of the following:
- chiral Schiff base ligands (R)/(S)-2-amino-3-(((1-hydroxypropan-2-yl)imino)methyl)-4H-chromen-4-one
- hydrazone derivatives, required for the synthesis of heterocyclic Schiff′s bases having antimicrobial activity
- N-{4-[(6-chloro-4-oxo-4H-chromen-3-ylmethylene)amino]phenyl}-3,5-dimethyl-benzofuran-2-carboxamide
- N-{4-[(6-chloro-4-oxo-4H-chromen-3-ylmethylene)amino]phenyl}-1,4-dihydro-chromono [2,3-b]pyrrole-2-carboxamide
- 6-chloro-3-{[4-(2-thioxo-3,6-dihydro-2H-1,3,4-thiadiazin-5-ylamino)phenylimino] methyl}- 4-oxo-4H -chromene
- 3-(2-amino-4-oxo-4H-chromen-3-yl)methylidene)-6- ethyl-2H-pyrano[3,2-c]quinolone-2,4,5(3H,6H)trione
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis and antimicrobial activity of some new heterocyclic Schiff bases derived from 2-amino-3-formylchromone.
Ibrahim MA and El-Mahdy KM.
Phosphorus, Sulfur, and Silicon and the Related Elements, 184(11), 2945-2958 (2009)
Novel heterocyclic derivatives of pyrano [3, 2-c] quinolinone from 3-(1-ethy1-4-hydroxy-2-oxo-2 (1H)-quinolin-3-yl)-3-oxopropanoic acid.
Ibrahim MA, et al.
European Journal of Chemistry, 1(3), 195-199 (2010)
Synthesis of some new 4-oxo-4H-chromene derivatives bearing nitrogen heterocyclic systems as antifungal agents.
Ali T E-S, et al.
Turkish Journal of Chemistry, 32(3), 365-374 (2008)
Farukh Arjmand et al.
Chirality, 24(12), 977-986 (2012-09-25)
Novel chiral Schiff base ligands (R)/(S)-2-amino-3-(((1-hydroxypropan-2-yl)imino)methyl)-4H-chromen-4-one (L(1) and L(2)) derived from 2-amino-3-formylchromone and (R/S)-2-amino-1-propanol and their Cu(II)/Zn(II) complexes (R1, S1, R2, and S2) were synthesized. The complexes were characterized by elemental analysis, infrared (IR), hydrogen ((1) H) and carbon ((13)C)
Farukh Arjmand et al.
Journal of photochemistry and photobiology. B, Biology, 103(2), 166-179 (2011-04-05)
New Schiff base ligand L derived from the condensation reaction of 2-amino-3-formylchromone with (R)-2-amino-2-phenylethanol was synthesized and characterized which involves combination element of ammine functionality and naturally occurring heterocyclic chromone, 4H-benzopyran-4-one. Subsequently, their complexes 1 and 2 with Cu(NO₃)₂ and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service