340480
trans,trans-Farnesyl acetate
95%
Synonym(s):
(E,E)-3,7,11-Trimethyl-2,6,10-dodecatrien-1-ol acetate, (E,E)-3,7,11-Trimethyl-2,6,10-dodecatrien-1-yl acetate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
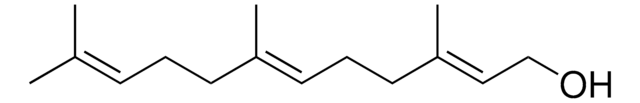
CH3CO2CH2CH=C(CH3)CH2CH2CH=C(CH3)CH2CH2CH=C(CH3)2
CAS Number:
Molecular Weight:
264.40
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
liquid
refractive index
n20/D 1.477 (lit.)
bp
115-125 °C/0.3 mmHg (lit.)
density
0.914 g/mL at 25 °C (lit.)
functional group
ester
SMILES string
CC(=O)OC\C=C(/C)CC\C=C(/C)CC\C=C(\C)C
InChI
1S/C17H28O2/c1-14(2)8-6-9-15(3)10-7-11-16(4)12-13-19-17(5)18/h8,10,12H,6-7,9,11,13H2,1-5H3/b15-10+,16-12+
InChI key
ZGIGZINMAOQWLX-NCZFFCEISA-N
General description
trans,trans-Farnesyl acetate is one of the most odor-active or key compounds of Hyuganatsu aroma in Hyuganatsu (Citrus tamurana Hort. ex Tanaka) peel oil. Asymmetric dihydroxylation of trans,trans-farnesyl acetate using the new Sharpless′ ligand has been studied.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
H S Choi et al.
Journal of agricultural and food chemistry, 49(5), 2404-2408 (2001-05-23)
The volatile components of Hyuganatsu (Citrus tamurana Hort. ex Tanaka) peel oil, isolated by cold-pressing, were investigated by chemical and sensory analyses. According to chemical analysis by GC and GC-MS, limonene (84.0%) was the most abundant compound, followed by gamma-terpinene
Asymmetric dihydroxylation of geranyl, neryl and< i> trans, trans</i>-farnesyl acetates.
Vidari G, et al.
Tetrahedron Letters, 34(40), 6485-6488 (1993)
Fangfang Zeng et al.
Insect biochemistry and molecular biology, 113, 103213-103213 (2019-08-24)
Mosquitoes rely heavily on the olfactory system to find a host for a bloodmeal, plants for a source of energy and suitable sites for oviposition. Here, we examined a cluster of eight odorant receptors (ORs), which includes one OR, CquiOR1
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service