301558
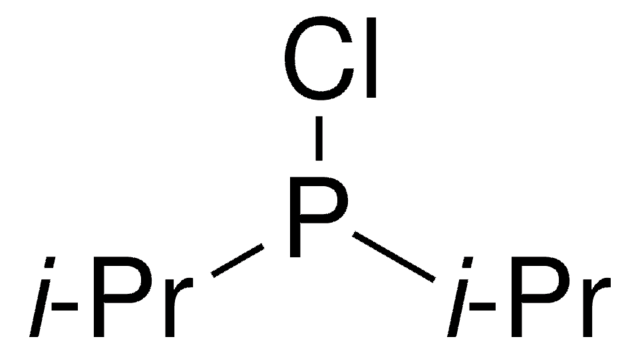
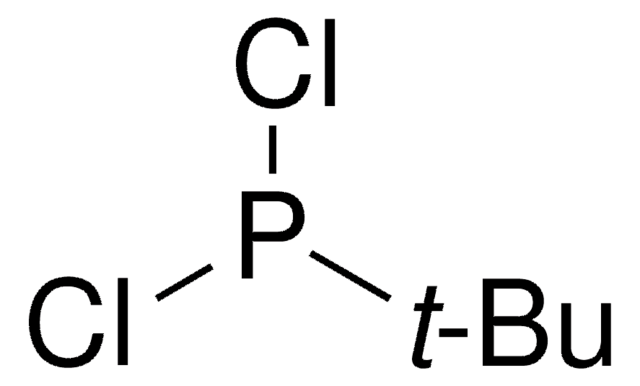
Di-tert-butylchlorophosphine
96%
Synonym(s):
P, P-bis(1 1-dimethylethyl), Phosphinous chloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
[(CH3)3C]2PCl
CAS Number:
Molecular Weight:
180.66
MDL number:
UNSPSC Code:
12352001
PubChem Substance ID:
Recommended Products
Quality Level
Assay
96%
form
liquid
reaction suitability
reagent type: ligand
reaction type: Suzuki-Miyaura Coupling
refractive index
n20/D 1.482 (lit.)
bp
48 °C/3 mmHg (lit.)
density
0.951 g/mL at 25 °C (lit.)
functional group
phosphine
SMILES string
CC(C)(C)P(Cl)C(C)(C)C
InChI
1S/C8H18ClP/c1-7(2,3)10(9)8(4,5)6/h1-6H3
InChI key
MCRSZLVSRGTMIH-UHFFFAOYSA-N
Related Categories
General description
Di-tert-butylchlorophosphine belongs to the class of phosphine ligands. It is used for cross-coupling reactions because of the flexibility of its electronic and steric properties. It plays a key role in stabilizing and activating the central metal atom and is used in reactions such as transition metal-catalyzed C-O, C-N, and C-C bond-forming reactions.
Application
Di-tert-butylchlorophosphine can be used as a ligand in:
- The Pd-catalyzed amination reaction with aryl halides.
- The Pd-catalyzed Suzuki-Miyaura cross-coupling of arylboronic acids with aryl bromides and chlorides.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
143.6 °F - closed cup
Flash Point(C)
62 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
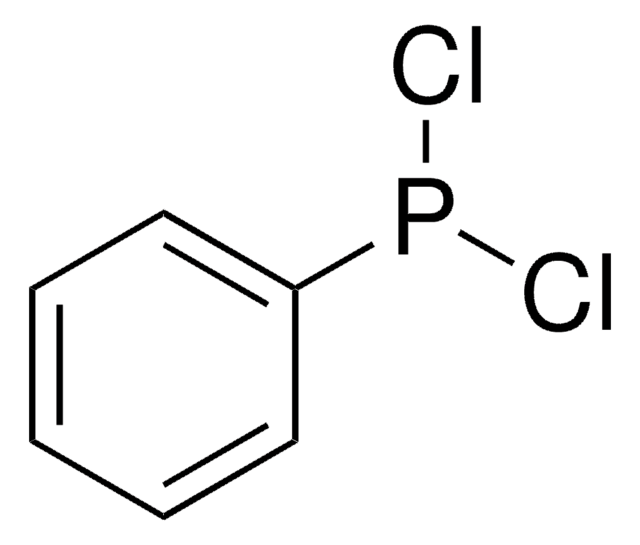
Jesudoss V Kingston et al.
The Journal of organic chemistry, 72(8), 2816-2822 (2007-03-24)
Pro-azaphosphatrane 1a [P(iBuNCH2CH2)3N] reacts with iodine under mild conditions to give [IP(iBuNCH2CH2)3N]I in excellent yield, which on subsequent reaction with ammonia followed by deprotonation with KOtBu provided HN=P(iBuNCH2CH2)3N (3a) in quantitative yield. Reaction of 3a with R'2PCl afforded sterically bulky
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 301558-100G | 4061838126412 |
| 301558-1G | |
| 301558-25G | 4061826665466 |
| 301558-5G | 4061826665473 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service