All Photos(1)
About This Item
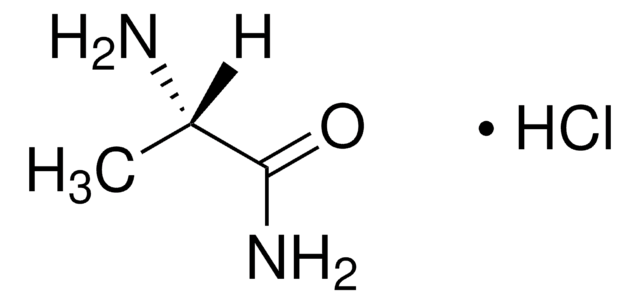
Linear Formula:
(CH3)2CHCH2CH(NH2)CONH2·HCl
CAS Number:
Molecular Weight:
166.65
Beilstein:
4237021
EC Number:
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
optical activity
[α]25/D +10°, c = 5 in H2O
reaction suitability
reaction type: solution phase peptide synthesis
mp
254-256 °C (lit.)
application(s)
peptide synthesis
SMILES string
Cl.CC(C)C[C@H](N)C(N)=O
InChI
1S/C6H14N2O.ClH/c1-4(2)3-5(7)6(8)9;/h4-5H,3,7H2,1-2H3,(H2,8,9);1H/t5-;/m0./s1
InChI key
VSPSRRBIXFUMOU-JEDNCBNOSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Claudio Jösch et al.
Biological chemistry, 384(2), 213-218 (2003-04-05)
Cysteinylglycine hydrolysis is a step in the metabolism of glutathione and glutathione S-conjugates. We had previously observed that in rat liver the enzymatic activity is predominantly located in the cytosol. Here we demonstrate that cytosolic leucyl aminopeptidase (EC 3.4.11.1) is
Measurement of carprofen enantiomer concentrations in plasma and urine using L-leucinamide as the chiral coupling component.
H Spahn et al.
Journal of chromatography, 433, 331-338 (1988-12-09)
HTS, chemical hybridization, and drug design identify a chemically unique antituberculosis agent-coupling serendipity and rational approaches to drug discovery.
Jialin Mao et al.
ChemMedChem, 2(6), 811-813 (2007-04-25)
B R Madan et al.
Research communications in chemical pathology and pharmacology, 58(3), 393-396 (1987-12-01)
L-leucinamide hydrochloride, an amino acid derivative, was found to share the ability of phenylbutazone in attenuating the phlogistic response induced by intraplantar injection of formaldehyde and nystatin in the unanesthetized rat. In the granuloma pouch induced by the injection of
E A Permiakov et al.
Bioorganicheskaia khimiia, 28(1), 11-15 (2002-03-06)
The kinetics of the reaction of Boc-Xaa fluorophenyl esters (where Xaa = Ala, Val, Phe, Ser, Leu, Gly, Met, Pro, or Ile) with leucinamide was studied measuring changes in the fluorescence emission at 375 nm of the fluorophenyl chromophore accompanying
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service