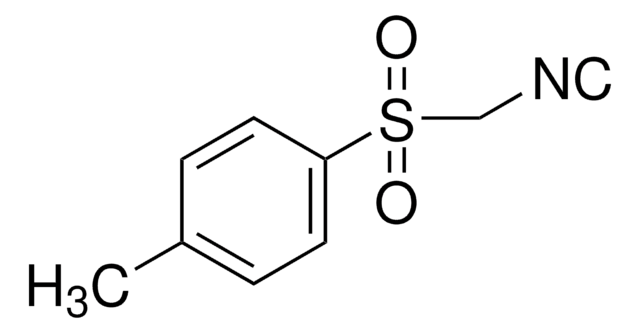
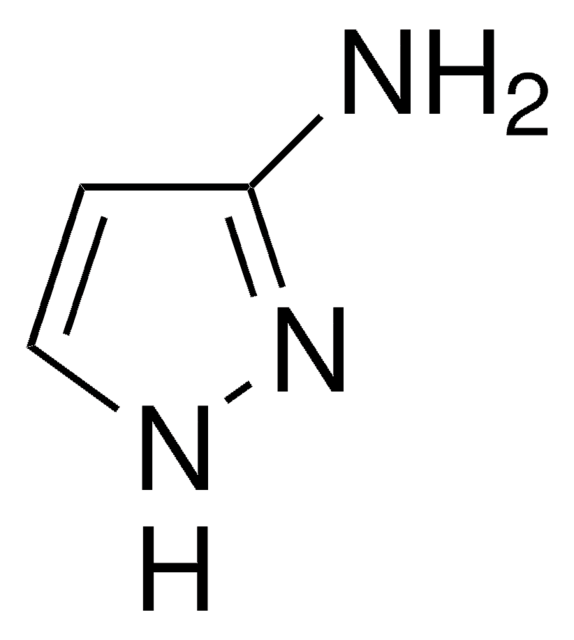
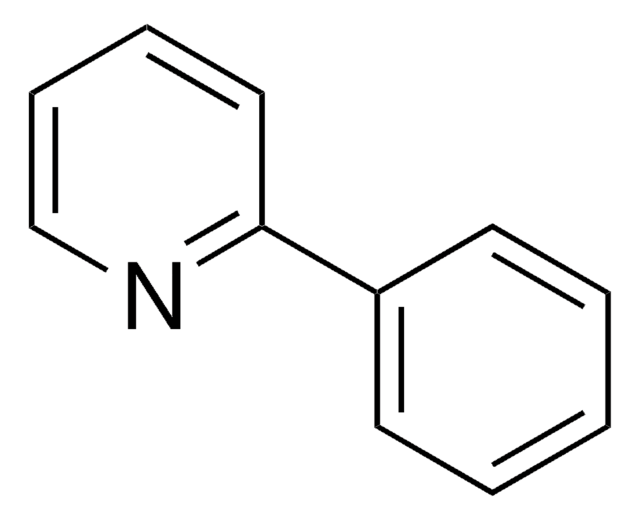
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H8N2
CAS Number:
Molecular Weight:
144.17
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
liquid
refractive index
n20/D 1.596 (lit.)
bp
141-142 °C/30 mmHg (lit.)
density
1.091 g/mL at 25 °C (lit.)
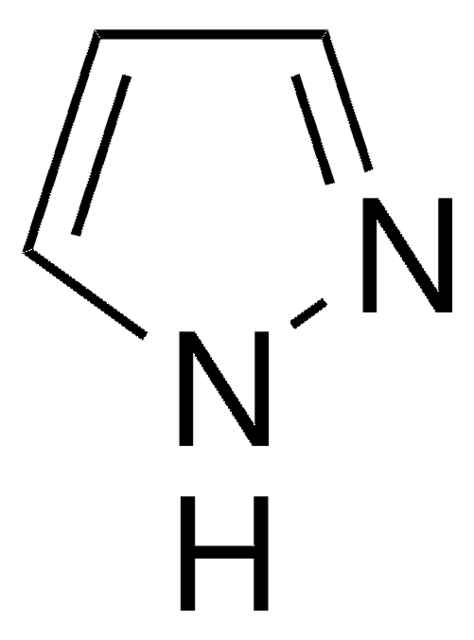
SMILES string
c1ccc(cc1)-n2cccn2
InChI
1S/C9H8N2/c1-2-5-9(6-3-1)11-8-4-7-10-11/h1-8H
InChI key
WITMXBRCQWOZPX-UHFFFAOYSA-N
General description
1-Phenylpyrazole undergoes cyclometallation with rhodium trichloride to yield rac-di(μ-chloro)tetrakis[2-(pyrazol-1-yl)phenyl-C1,N2′]dirhodium. The activation of the C-H bond of 1-phenylpyrazole autocatalyzed by the co-product HOAc was studied.
Application
1-Phenylpyrazole has been used:
- in the preparation of 4,5-diphenylpyrazolo[1,5-a]quinoline, 1-(1,2,3,4-tetraphenylnaphthalen-5-yl)pyrazole and 1-(1,2,3,4,5,6,7,8-octaphenylanthracen-9-yl)pyrazole
- as cyclometallated ligand in the preparation of new heteroleptic iridium(III) complexes
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Jian-ning Yu et al.
Guang pu xue yu guang pu fen xi = Guang pu, 30(9), 2424-2427 (2010-11-26)
New heteroleptic iridium(III) complexes (ppz)2Ir(LX), which consist of two cyclometalated ligands ppz(1-phenylpyrazole) together with an ancillary ligand LX (LX= 2-(2'-hydroxylphenyl)benzothiazole (BTZ), 2-(3'-methyl-2'-hydroxylphenyl) benzothiazole (3-MeBTZ), 2-(4'-methyl-2'-hydroxylphenyl) benzothiazole (4-MeBTZ) and 2-(4'-Trifluoromethyl-2'hydroxylphenyl) benzothiazole (4-tfmBTZ)), were synthesized and characterized. The molecular structures and photophysical
Cyclometallated compounds: VII. X-Ray crystal structure of the product of cyclometallation of 1-phenylpyrazole with rhodium trichloride.
Steel PJ.
Journal of Organometallic Chemistry, 408(3), 395-402 (1991)
Indira Fabre et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 19(23), 7595-7604 (2013-04-19)
The activation of the C-H bond of 1-phenylpyrazole (2) and 2-phenyl-2-oxazoline (3) by [Ru(OAc)2(p-cymene)] is an autocatalytic process catalyzed by the co-product HOAc. The reactions are indeed faster in the presence of acetic acid and water but slower in the
Nobuyoshi Umeda et al.
The Journal of organic chemistry, 76(1), 13-24 (2010-12-15)
The direct oxidative coupling of phenylazoles with internal alkynes proceeds efficiently in the presence of a rhodium catalyst and a copper oxidant accompanied by double or quadruple C-H bond cleavages. Thus, as a representative example, 4,5-diphenylpyrazolo[1,5-a]quinoline, 1-(1,2,3,4-tetraphenylnaphthalen-5-yl)pyrazole, and 1-(1,2,3,4,5,6,7,8-octaphenylanthracen-9-yl)pyrazole can
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service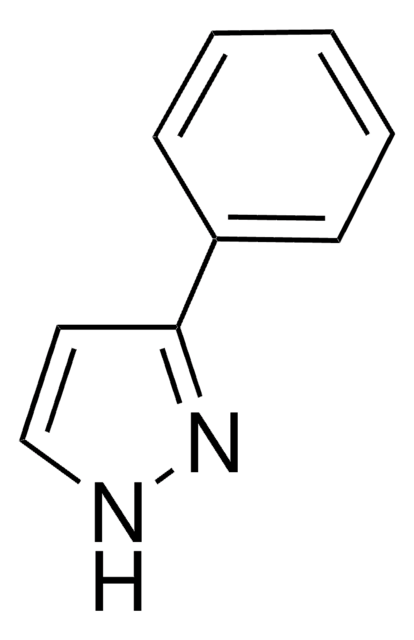
![Benzo[h]quinoline 97%](/deepweb/assets/sigmaaldrich/product/structures/344/715/928932d2-4ca4-4402-b56c-85a80100ce17/640/928932d2-4ca4-4402-b56c-85a80100ce17.png)