All Photos(1)
About This Item
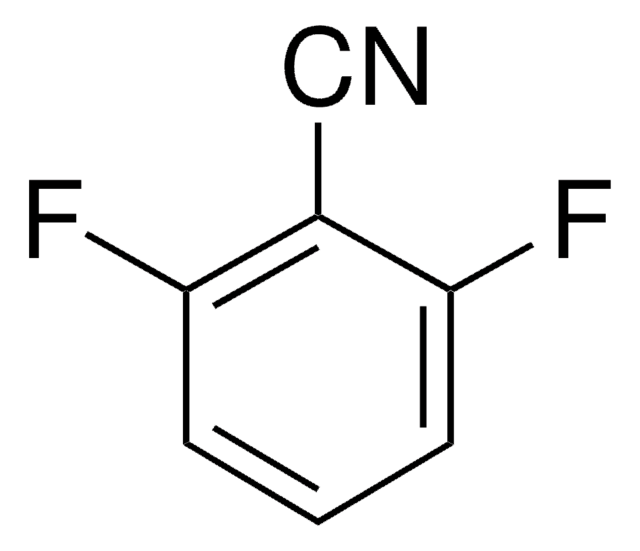
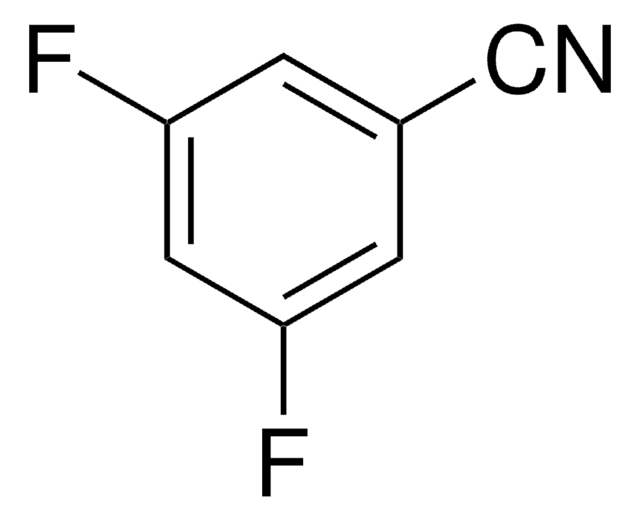
Linear Formula:
F2C6H3CN
CAS Number:
Molecular Weight:
139.10
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
refractive index
n20/D 1.4882 (lit.)
density
1.254 g/mL at 25 °C (lit.)
functional group
fluoro
nitrile
SMILES string
Fc1cccc(C#N)c1F
InChI
1S/C7H3F2N/c8-6-3-1-2-5(4-10)7(6)9/h1-3H
InChI key
GKPHNZYMLJPYJJ-UHFFFAOYSA-N
General description
Electronic transitions of 2,3-difluorobenzonitrile has been studied by photoacoustic spectroscopy.
Application
2,3-Difluorobenzonitrile has been used in the preparation of difluorophenyl-1,2,3,5-dithiadiazolyl and cyanophenoxazines.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
167.0 °F - closed cup
Flash Point(C)
75 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
The effect of fluorinated aryl substituents on the crystal structures of 1, 2, 3, 5-dithiadiazolyl radicals.
Clarke CS, et al.
CrystEngComm, 12(1), 172-185 (2010)
Cyano-activated fluoro displacement reactions in the synthesis of cyanophenoxazines and related compounds.
Eastmond GC, et al.
New. J. Chem., 25(3), 385-390 (2001)
M Alcolea Palafox et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 57(12), 2373-2389 (2002-01-05)
Geometry, vibrational frequencies, atomic charges and several thermodynamic parameters (the total energy, the zero point energy, the rotational constants and the room temperature entropy) were calculated using ab initio quantum chemical methods for 2,3-difluorobenzonitrile molecule. The results were compared with
Two electronic transitions in the near-UV region of six isomeric difluorobenzonitriles-I.
Ramu K, et al.
Spectrochimica Acta. Part A, Molecular and Biomolecular Spectroscopy, 49(2), 223-236 (1993)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service