All Photos(1)
About This Item
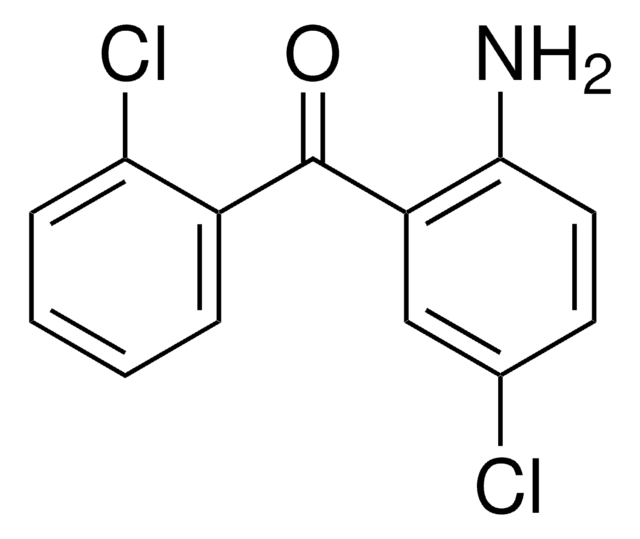
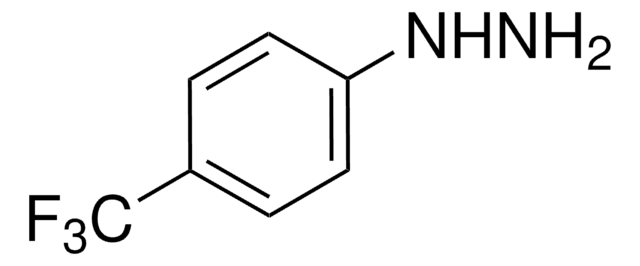
Linear Formula:
H2NC6H3(NO2)C(O)C6H5
CAS Number:
Molecular Weight:
242.23
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥98%
form
powder
mp
166-168 °C (lit.)
functional group
ketone
nitro
phenyl
SMILES string
Nc1ccc(cc1C(=O)c2ccccc2)[N+]([O-])=O
InChI
1S/C13H10N2O3/c14-12-7-6-10(15(17)18)8-11(12)13(16)9-4-2-1-3-5-9/h1-8H,14H2
InChI key
PZPZDEIASIKHPY-UHFFFAOYSA-N
General description
FT-IR and Raman spectra of 2-amino-5-nitrobenzophenone (ANBP) has been reported.
Application
2-Amino-5-nitrobenzophenone was used in the synthesis of [5-(4-nitrophenyl)-2-furyl]acrylic acid substituted benzophenone (anti-malarial agent).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Jochen Wiesner et al.
Bioorganic & medicinal chemistry letters, 13(3), 361-363 (2003-02-05)
We have developed the [5-(4-nitrophenyl)-2-furyl]acrylic acid substituted benzophenone 4g as a novel lead for anti-malarial agents. Here, we demonstrated that the acyl residue at the 2-amino group of the benzophenone core structure has to be a phenylacetic acid substructure substituted
V Balachandran et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 118, 835-846 (2013-10-25)
The FT-IR and Raman spectra of 2-amino-5-nitrobenzophenone (ANBP) molecule have been recorded using Brucker IFS 66 V spectrometer in the range of 4000-100 cm(-1). The molecular geometry and vibrational frequencies in the ground state are calculated using the Hartree-Fock (HF)
P J Cox et al.
International journal of pharmaceutics, 194(2), 147-153 (2000-02-29)
The role of single crystal diffraction in the quantitative determination of polymorphism is demonstrated by the examination of three compounds. Two polymorphs were found for each of the compounds bis(2-nitrophenyl) trisulphide (1), 2-amino-5-nitrobenzophenone (2) and bis(2-nitrophenyl) sulphide (3). Only in
Koichi Saito et al.
Chemical & pharmaceutical bulletin, 69(3), 258-264 (2021-03-02)
The degradation behavior of eight benzodiazepines (BZPs): alprazolam, etizolam, diazepam, triazolam, nitrazepam (NZP), flunitrazepam (FNZ), bromazepam, and lorazepam, in artificial gastric juice was monitored by a LC/photodiode array detector (PDA) to estimate their pharmacokinetics in the stomach. For drugs that
T Inoue et al.
Journal of chromatography, 339(1), 163-169 (1985-04-12)
A method for the direct quantitative densitometry of nitrazepam and its main metabolites (7-aminonitrazepam, 7-acetamidonitrazepam and 2-amino-5-nitrobenzophenone) in urine was developed. The unchanged drug and its metabolites were extracted with benzene-dichloromethane (4:1), subjected to thin-layer chromatography, and determined by direct
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service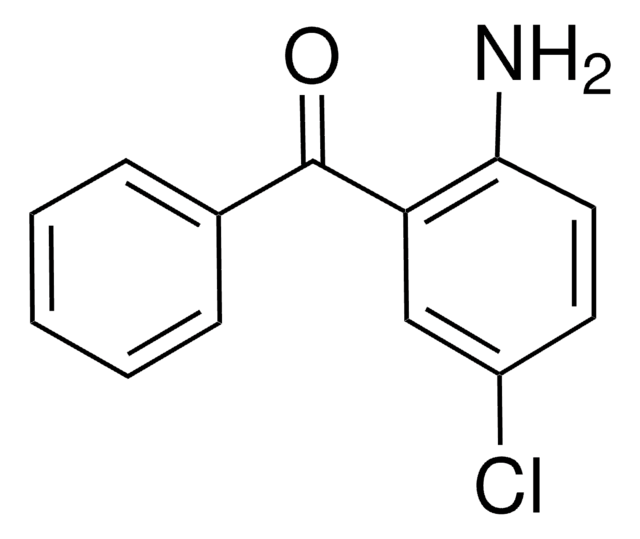

![BICYCLO[2.2.1]HEPT-5-ENE-2-CARBONITRILE AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/423/632/9f9c4d06-10c1-4935-ac11-4f0e71373dd4/640/9f9c4d06-10c1-4935-ac11-4f0e71373dd4.png)