187070
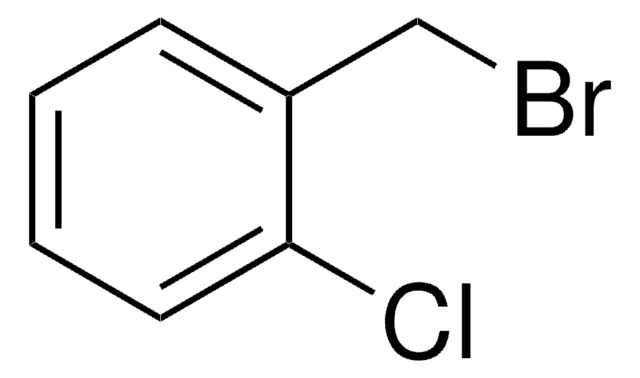
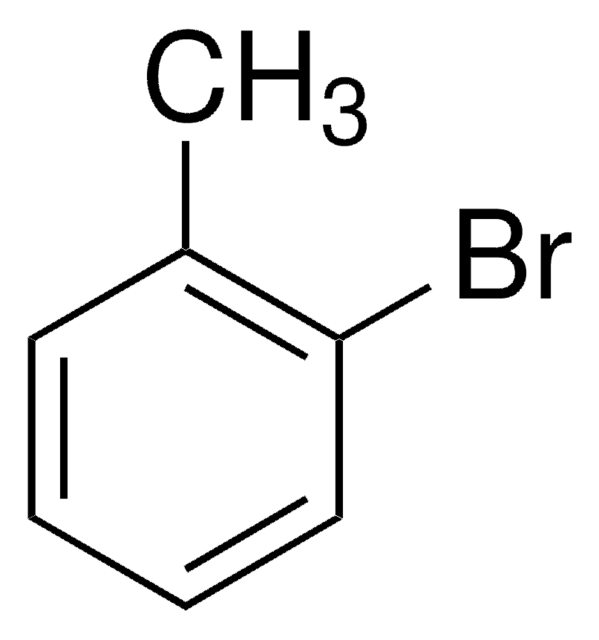
2-Bromobenzyl bromide
98%
Synonym(s):
α,2-Dibromotoluene
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
BrC6H4CH2Br
CAS Number:
Molecular Weight:
249.93
Beilstein:
971015
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
refractive index
n20/D 1.619 (lit.)
bp
129 °C/19 mmHg (lit.)
mp
29-32 °C (lit.)
solubility
dioxane: soluble 1 g/10 mL, clear, colorless
functional group
bromo
SMILES string
BrCc1ccccc1Br
InChI
1S/C7H6Br2/c8-5-6-3-1-2-4-7(6)9/h1-4H,5H2
InChI key
LZSYGJNFCREHMD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2-Bromobenzyl bromide is a reagent used to protect ketones and aldehydes in their less reactive alcohol oxidation states and as a coupling component in various reactions.
Application
2-Bromobenzyl bromide was used in the synthesis of:
- substituted quinazolines and 1,2,3,4-tetrahydroquinazolines
- 2- and 3-substituted indenes
- tris-2-bromotribenzylamine
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis and structural studies of tris-2-chlorobenzylamine and tris-2-bromobenzylamine.
Chen Q, , et al.
Journal of Chemical Crystallography, 35(3), 177-181 (2005)
Efficient synthesis of 2-and 3-substituted indenes from 2-bromobenzyl bromide through an enolate alkylation/Cr (II)/Ni (II)-mediated carbonyl addition sequence.
Halterman RLand Zhu C.
Tetrahedron Letters, 40(42), 7445-7448 (1999)
Xuesen Fan et al.
Chemistry, an Asian journal, 9(3), 739-743 (2014-01-01)
An efficient synthesis of diversely substituted quinazolines and 1,2,3,4-tetrahydroquinazolines through copper-catalyzed tandem reactions of the readily available 2-bromobenzyl bromides, aldehydes, and aqueous ammonia or amines has been developed. By using ammonia and simple aliphatic amines as the nitrogen source, the
Prachi Singh et al.
SLAS discovery : advancing life sciences R & D, 22(4), 440-446 (2017-03-23)
Analysis of interactions between molecules is of fundamental importance in life science research. In this study, we applied weak affinity chromatography, based on high-performance liquid chromatography and mass spectrometry, as a powerful tool for direct analysis of the components of
William L Scott et al.
Journal of combinatorial chemistry, 11(1), 14-33 (2008-12-25)
Distributed Drug Discovery (D(3)) proposes solving large drug discovery problems by breaking them into smaller units for processing at multiple sites. A key component of the synthetic and computational stages of D(3) is the global rehearsal of prospective reagents and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service