160660
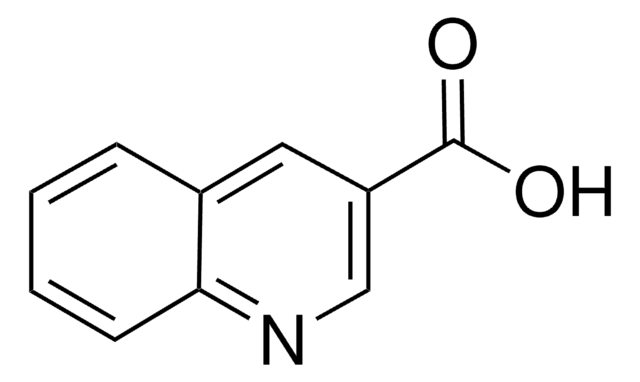
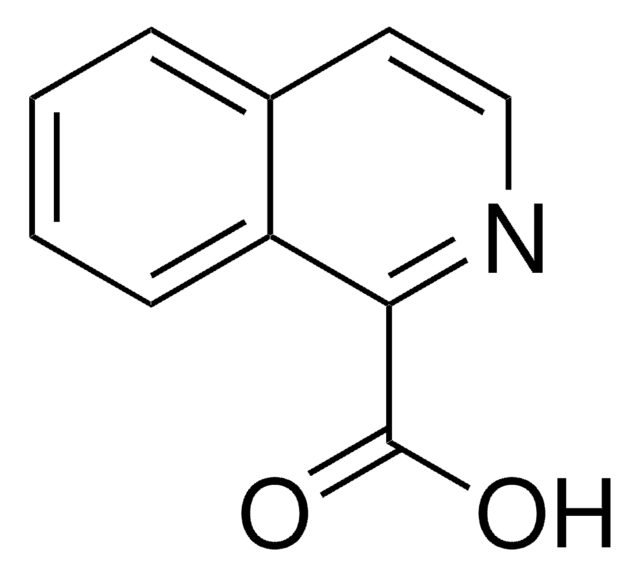
Quinaldic acid
98%
Synonym(s):
2-Quinolinecarboxylic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H7NO2
CAS Number:
Molecular Weight:
173.17
Beilstein:
126322
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
156-158 °C (lit.)
functional group
carboxylic acid
SMILES string
OC(=O)c1ccc2ccccc2n1
InChI
1S/C10H7NO2/c12-10(13)9-6-5-7-3-1-2-4-8(7)11-9/h1-6H,(H,12,13)
InChI key
LOAUVZALPPNFOQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Quinaldic acid is also referred as quinoline-2-carboxylic acid. Microwave-assisted preparation of substituted anilides of quinaldic acid has been reported. It inhibits the oxidation of pyruvate, α-ketoglutarate, glutamate and citrate in rat liver mitochondria. Quinaldic acid is a metabolite of tryptophan degradation and inhibits the gluconeogenesis in perfused livers.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
S Fetzner et al.
Biological chemistry Hoppe-Seyler, 374(6), 363-376 (1993-06-01)
Serratia marcecens 2CC-1 utilizes quinaldic acid (quinoline 2-carboxylic acid) as sole source of carbon, nitrogen and energy. Growth of strain 2CC-1 on quinaldic acid as well as on nicotinic acid and hypoxanthine was inhibited completely by the molybdate antagonist tungstate
Mode of action of hypoglycemic agents. 3. Studies on 5-methoxy indole-2-carboxylic acid and quinaldic acid.
J Reed et al.
The Journal of biological chemistry, 245(20), 5297-5303 (1970-10-25)
Total synthesis of thiostrepton, part 2: construction of the quinaldic acid macrocycle and final stages of the synthesis.
K C Nicolaou et al.
Angewandte Chemie (International ed. in English), 43(38), 5092-5097 (2004-09-17)
R M Epand
Medical hypotheses, 9(2), 207-213 (1982-08-01)
Ergothioneine is believed not to be synthesized by man but it accumulates to high concentrations in some mammalian cells as a result of dietary intake. Ergothioneine is known to chelate divalent metal ions with high affinity. Other substances which are
Stereocontrolled synthesis of the quinaldic acid macrocyclic system of thiostrepton.
K C Nicolaou et al.
Angewandte Chemie (International ed. in English), 41(11), 1937-1940 (2002-06-03)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service