154180
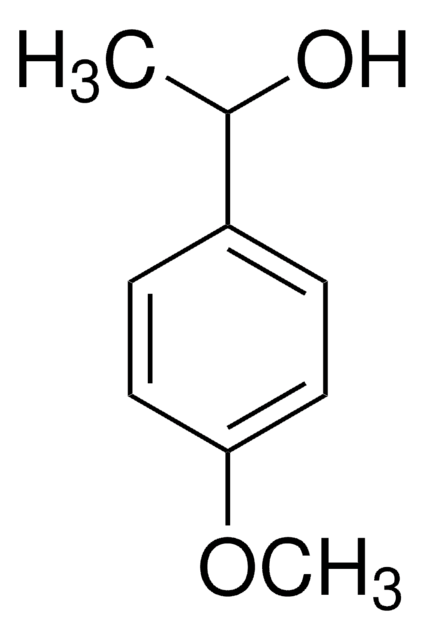
4-Methoxyphenethyl alcohol
99%
Synonym(s):
2-(4-Methoxyphenyl)ethanol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3OC6H4CH2CH2OH
CAS Number:
Molecular Weight:
152.19
Beilstein:
2043563
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
solid
bp
334-336 °C (lit.)
mp
26-28 °C (lit.)
functional group
hydroxyl
SMILES string
COc1ccc(CCO)cc1
InChI
1S/C9H12O2/c1-11-9-4-2-8(3-5-9)6-7-10/h2-5,10H,6-7H2,1H3
InChI key
AUWDOZOUJWEPBA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
(R)-1-(4-methoxyphenyl)ethanol {(R)-MOPE, 4-Methoxyphenethyl alcohol, 1-(4-methoxyphenyl)ethanol } is formed during the biocatalytic anti-Prelog enantioselective reduction of 4-methoxyacetophenone (MOAP) using immobilized Trigonopsis variabilis AS2.
Application
4-Methoxyphenethyl alcohol was used as an internal standard in the fluorous biphasic catalysis reaction.
4-Methoxyphenethyl alcohol was used in the preparation of 4-(2-iodoethyl)phenol, by refluxing it with 47% hydriodic acid. It may be used in the preparation of (2R*,4R*)-1-n-butyl-2-methyl-4-(2-oxopyrrolidin-1-yl)-6-methoxy-1,2,3,4-tetrahydroquinoline and (2R*,4S*)-1-n-butyl-2-methyl-4-(2-oxopyrrolidin-1-yl)-6-methoxy-1,2,3,4- tetrahydroquinoline.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Biocatalytic anti-Prelog stereoselective reduction of 4'-methoxyacetophenone to (R)-1-(4-methoxyphenyl) ethanol with immobilized Trigonopsis variabilis AS2. 1611 cells using an ionic liquid-containing medium.
Lou W-Y, et al.
Green Chemistry, 11(9), 1377-1384 (2009)
Synthesis of 5-(ω-sulfhydrylalkyl) salicylaldehydes as precursors for the preparation of alkanethiol-modified metal salens.
Ji C and Peters DG
Tetrahedron Letters, 42(35), 6065-6067 (2001)
An asymmetric catalytic carbon? carbon bond formation in a fluorous biphasic system based on perfluoroalkyl-BINOL.
Tian Y and Chan KS.
Tetrahedron Letters, 41(45), 8813-8816 (2000)
Reactions of Azides with Electrophiles: New Methods for the Generation of Cationic 2-Azabutadienes. Synthesis of 1, 2, 3, 4-Tetrahydroquinolines and 1, 2-Dihydroquinolines via a Hetero Diels-Alder Reaction.
Pearson WH and Fang WK
Israel J. Chem., 37(1), 39-46 (1997)
Min Kyung Song et al.
Journal of agricultural and food chemistry, 67(7), 2028-2035 (2019-01-31)
Caffeic acid phenethyl ester (CAPE) is an ester of a hydroxycinnamic acid (phenylpropanoid) and a phenylethanoid (2-phenylethanol; 2-PE), which has long been used in traditional medicine. Here, we synthesized 54 hydroxycinnamic acid-phenylethanoid esters by feeding 64 combinations of hydroxycinnamic acids
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 154180-50G | |
| 154180-10G | 4061838346186 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service