15406
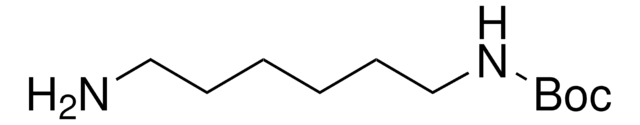
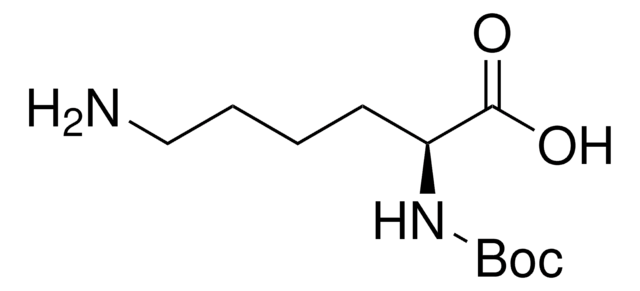
N-Boc-cadaverine
≥97.0% (NT)
Synonym(s):
N-Boc-1,5-diaminopentane, tert-Butyl N-(5-aminopentyl)carbamate
About This Item
Recommended Products
Quality Level
Assay
≥97.0% (NT)
reaction suitability
reagent type: cross-linking reagent
refractive index
n20/D 1.460
density
0.972 g/mL at 20 °C (lit.)
functional group
Boc
amine
SMILES string
NCCCCCNC(OC(C)(C)C)=O
InChI
1S/C10H22N2O2/c1-10(2,3)14-9(13)12-8-6-4-5-7-11/h4-8,11H2,1-3H3,(H,12,13)
InChI key
DPLOGSUBQDREOU-UHFFFAOYSA-N
Related Categories
Application
- Synthesis of of a supermacrocycle that self-assemble to form organic nanotubes.
- Preparation of water-soluble unsymmetrical sulforhodamine fluorophores from monobrominated sulfoxanthene dye.
- Synthesis of functionalized porphyrins as biocompatible carrier system for photodynamic therapy (PDT).
Other Notes
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible, corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
228.2 °F - closed cup
Flash Point(C)
109.0 °C - closed cup
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
MRT - Mono-Boc-Protection of Diamines
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service





![2-[2-(Boc-amino)ethoxy]ethanol 97%](/deepweb/assets/sigmaaldrich/product/structures/413/416/884359e5-1cb4-4071-bb4f-28d9844db662/640/884359e5-1cb4-4071-bb4f-28d9844db662.png)
