134996
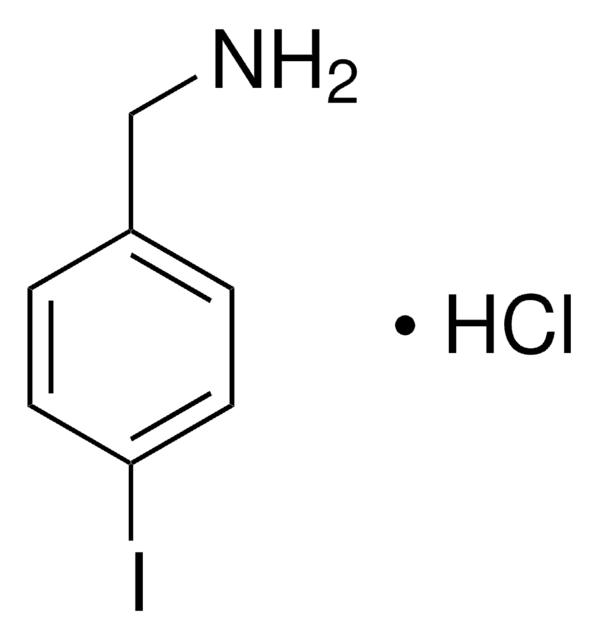
3-Iodobenzylamine hydrochloride
97%
Synonym(s):
(3-Iodophenyl)methanamine hydrochloride, m-Iodobenzylamine hydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
IC6H4CH2NH2 · HCl
CAS Number:
Molecular Weight:
269.51
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
188-190 °C (lit.)
SMILES string
Cl.NCc1cccc(I)c1
InChI
1S/C7H8IN.ClH/c8-7-3-1-2-6(4-7)5-9;/h1-4H,5,9H2;1H
InChI key
PYFDZOCGFHIRST-UHFFFAOYSA-N
Related Categories
Application
3-Iodobenzylamine hydrochloride was used as the starting reagent in the synthesis of N6-(3-iodobenzyl)-2-substituted-adenosine derivatives. It was used in the synthesis of 3′-C-methyl adenosine N6-substituted and N6/C-2 disubstituted derivatives and novel 2′-C-methyl analogues.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Repr. 2 - Resp. Sens. 1 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
H O Kim et al.
Journal of medicinal chemistry, 37(21), 3614-3621 (1994-10-14)
Adenosine derivatives bearing an N6-(3-iodobenzyl) group, reported to enhance the affinity of adenosine-5'-uronamide analogues as agonists at A3 adenosine receptors (J. Med. Chem. 1994, 37, 636-646), were synthesized starting from methyl beta-D-ribofuranoside in 10 steps. Binding affinities at A1 and
Loredana Cappellacci et al.
Journal of medicinal chemistry, 48(5), 1550-1562 (2005-03-04)
A number of 3'-C-methyl analogues of selective adenosine receptor agonists such as CPA, CHA, CCPA, 2'-Me-CCPA, NECA, and IB-MECA was synthesized to further investigate the subdomain of the receptor that binds the ribose moiety of the ligands. Affinity data at
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service