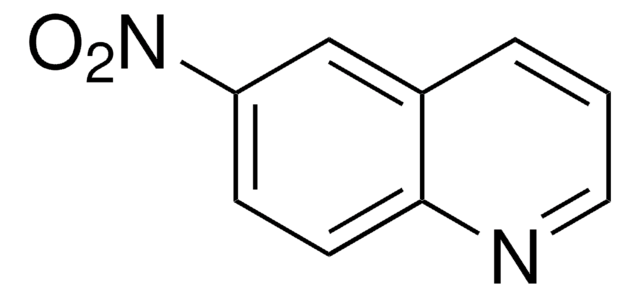
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H6N2O2
CAS Number:
Molecular Weight:
174.16
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
powder
mp
106-109 °C (lit.)
SMILES string
[O-][N+](=O)c1cccc2cnccc12
InChI
1S/C9H6N2O2/c12-11(13)9-3-1-2-7-6-10-5-4-8(7)9/h1-6H
InChI key
PYGMPFQCCWBTJQ-UHFFFAOYSA-N
General description
5-Nitroisoquinoline reacted with vinylmagnesium bromide to form a number of pyrroloisoquinolines. 5-Nitroisoquinoline derivatives (potential antimalarial drugs) were evaluated for mutagenic (MUT) and chromosome-damaging (CHR) activities by the Salmonella test.
Application
5-Nitroisoquinoline was used to prepare a number of pyrroloisoquinolines.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Margarita Vlachou et al.
European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences, 17(3), 139-143 (2002-10-24)
A number of pyrroloisoquinolines have been prepared by reaction of 5-nitroisoquinoline with vinylmagnesium bromide followed by N-alkylation with the appropriate 2-chloro-N,N-dialkylethylamine. Their cytotoxicity was evaluated in a number of ovarian cell lines and compared to their analogous isomeric pyrroloquinolines. Two
T Orsière et al.
Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association, 41(2), 275-290 (2002-12-14)
The mutagenic (MUT) and chromosome-damaging (CHR) activities of 22 potential antimalarial drugs (5-nitroisoquinoline derivatives) were evaluated by the Salmonella test and the cytokinesis-blocked micronucleus assay (CBMN). The Salmonella mutagenicity test was performed with and without metabolic activation (S9 mix) in
Nelson R Vinueza et al.
Physical chemistry chemical physics : PCCP, 20(33), 21567-21572 (2018-08-11)
Two previously unreported isomeric biradicals with a 1,4-radical topology, the 1,5-didehydroisoquinolinium cation and the 4,8-didehydroisoquinolinium cation, and an additional, previously reported isomer, the 4,5-didehydroisoquinolinium cation, were studied to examine the importance of the exact location of the radical sites on
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service