T8003
Trypsin from bovine pancreas
Type I, ~10,000 BAEE units/mg protein
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Recommended Products
type
Type I
form
solid
specific activity
~10,000 BAEE units/mg protein
mol wt
23.8 kDa
composition
protein, 90-100%
solubility
hydrochloric acid: soluble 1 mM, clear
foreign activity
Chymotrypsin ≤4 BTEE units/mg protein
shipped in
wet ice
storage temp.
−20°C
Looking for similar products? Visit Product Comparison Guide
Application
For trypsin digestion of peptides, use a ratio of about 1:100 to 1:20 for trypsin:peptide. The typical use for this product is in removing adherent cells from a culture surface. The concentration of trypsin necessary to dislodge cells from their substrate is dependent primarily on the cell type and the age of the culture. Trypsins have also been used for the re-suspension of cells during cell culture, in proteomics research for digestion of proteins and in various in-gel digestions. Additional applications include assessing crystallization by membrane-based techniques and in a study to determine that protein folding rates and yields can be limited by the presence of kinetic traps.
Biochem/physiol Actions
Trypsin cleaves peptides on the C-terminal side of lysine and arginine residues. The rate of hydrolysis of this reaction is slowed if an acidic residue is on either side of the cleavage site and hydrolysis is stopped if a proline residue is on the carboxyl side of the cleavage site. The optimal pH for trypsin activity is 7-9. Trypsin can also act to cleave ester and amide linkages of synthetic derivatives of amino acids. EDTA is added to trypsin solutions as a chelating agent that neutralizes calcium and magnesium ions that obscure the peptide bonds on which trypsin acts. Removing these ions increases the enzymatic activity.
Serine protease inhibitors, including DFP, TLCK, APMSF, AEBSEF, and aprotinin, amongst others, will inhibit Trypsin.
Serine protease inhibitors, including DFP, TLCK, APMSF, AEBSEF, and aprotinin, amongst others, will inhibit Trypsin.
Components
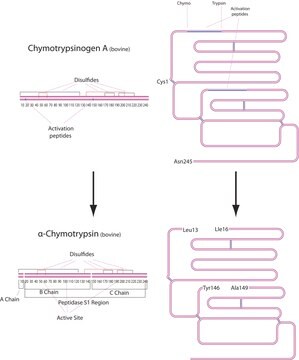
Trypsin consists of a single chain polypeptide of 223 amino acid residues, produced by the removal of the N-terminal hexapeptide from trypsinogen which is cleaved at the Lys - lle peptide bond. The sequence of amino acids is cross-linked by 6 disulfide bridges. This is the native form of trypsin, beta-trypsin. BETA-trypsin can be autolyzed, cleaving at the Lys - Ser residue, to produce alpha-trypsin. Trypsin is a member of the serine protease family.
Caution
Solutions in 1 mM HCl are stable for 1 year in aliquots and stored at -20°C. The presence of Ca2+ will also diminish the self-autolysis of trypsin and maintain its stability in solution. Trypsin will also retain most of its activity in 2.0 M urea, 2.0 M guanidine HCl, or 0.1% (w/v) SDS.
Unit Definition
One BAEE unit will produce a A253 of 0.001 per minute at pH 7.6 at 25°C using BAEE as a substrate.
Preparation Note
Soluble in 1 mM HCl at 1 mg/mL.
inhibitor
Product No.
Description
Pricing
substrate
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Resp. Sens. 1 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Geraldine Delbès et al.
Biology of reproduction, 80(2), 320-327 (2008-11-07)
Advances in treatment for testicular cancer that include the coadministration of bleomycin, etoposide, and cisplatin (BEP) have brought the cure rate to higher than 90%%. The goal of this study was to elucidate the impact of BEP treatment on gene
Renald A Blundell et al.
Experimental and molecular pathology, 79(1), 74-78 (2005-06-09)
Integrins are a family of cell surface glycoproteins that act as receptors for ECM proteins or for membrane bound counter-receptors on other cells. The integrin receptor family of vertebrates includes at least 16 distinct alpha subunits and at least 8
Antonio Jesús Vizcaíno et al.
Marine drugs, 18(6) (2020-06-24)
This piece of research evaluates the presence of protease inhibitors in the macroalga Ulva ohnoi and provides an initial overview of their mode of action. The ability of Ulva protease inhibitors to inhibit digestive proteases of three marine fish species
Curt Mazur et al.
JCI insight, 4(20) (2019-10-18)
Intrathecal (IT) delivery and pharmacology of antisense oligonucleotides (ASOs) for the CNS have been successfully developed to treat spinal muscular atrophy. However, ASO pharmacokinetic (PK) and pharmacodynamic (PD) properties remain poorly understood in the IT compartment. We applied multimodal imaging
Bahram Peivastegan et al.
BMC plant biology, 19(1), 262-262 (2019-06-19)
Stored potato (Solanum tuberosum L.) tubers are sensitive to wet conditions that can cause rotting in long-term storage. To study the effect of water on the tuber surface during storage, microarray analysis, RNA-Seq profiling, qRT-PCR and phytohormone measurements were performed
Protocols
Enzymatic Assay of Trypsin Inhibitor
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service