SRP6063
PIN1 human
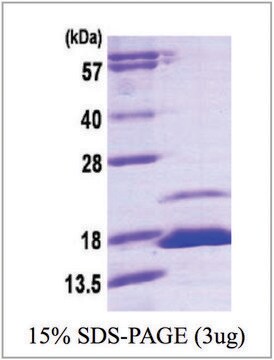
recombinant, expressed in E. coli, ≥95% (SDS-PAGE)
Synonym(s):
DOD, NIMA-interacting protein 1, PPIase Pin1, Rotamase Pin1, UBL5
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
biological source
human
recombinant
expressed in E. coli
Assay
≥95% (SDS-PAGE)
form
liquid
mol wt
18.2 kDa (163 aa)
packaging
pkg of 100 μg
NCBI accession no.
shipped in
dry ice
storage temp.
−70°C
Gene Information
human ... PIN1(5300)
General description
PIN1 (peptidylprolyl cis/trans isomerase, NIMA-interacting 1) is a peptidyl prolyl cis-trans isomerase. It is a conserved eukaryotic protein that contains an amino-terminal WW domain, which functions as a phosphorylated serine or threonine residue-binding site. Its C-terminal domain functions as the enzymatic domain, and is responsible for catalyzing the cis/trans isomerization of pSer/Thr-Pro bonds.
Biochem/physiol Actions
PIN1 (peptidylprolyl cis/trans isomerase, NIMA (never in mitosis A)-interacting 1) associates specifically with the phosphoserine-proline or phosphothreonine-proline residues immediately preceding proline (pSer/Thr-Pro), and promotes cis/trans isomerization of the peptide bond. This protein is involved in the amplification of the phosphorylation signaling, and is involved in catalyzing target dephosphorylation, control of protein stability and ubiquitination. It also mediates the cellular localization of its target proteins. It is highly up-regulated in multiple types of cancers, such as breast and prostate cancer, and is essential for the functionality and cross-talk of tumorigenic pathways. It is also implicated in Alzheimer′s disease (AD) and asthma.
Physical form
1 mg/mL solution in 20 mM Tris-HCl buffer (pH 7.5) containing 100 mM NaCl, 5 mM DTT, 20% glycerol.
Preparation Note
Centrifuge the vial prior to opening.
Other Notes
MADEEKLPPG WEKRMSRSSG RVYYFNHITN ASQWERPSGN SSSGGKNGQG EPARVRCSHL LVKHSQSRRP SSWRQEKITR TKEEALELIN GYIQKIKSGE EDFESLASQF SDCSSAKARG DLGAFSRGQM QKPFEDASFA LRTGEMSGPV FTDSGIHIIL RTE
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Guo-Liang Huang et al.
Cell death & disease, 7(12), e2571-e2571 (2016-12-30)
The function of activating transcription factor 1 (ATF1) and the mechanism about why ATF1 was over-phosphorylated in nasopharyngeal carcinoma (NPC) progression is completely undiscovered. In this study, a series of experiments both in vitro and in vivo were used to
Pin1 is related with clinical stage of papillary thyroid carcinoma.
Jiang L et al
World Journal of Surgical Oncology, 14, 95-95 (2016)
Peptidyl-Prolyl cis/trans Isomerase NIMA-Interacting 1 as a Therapeutic Target in Hepatocellular Carcinoma.
Kim G et al
Biological & Pharmaceutical Bulletin, 38(7), 975-979 (2015)
Pin1 induction in the fibrotic liver and its roles in TGF-β1 expression and Smad2/3 phosphorylation.
Jin Won Yang et al.
Journal of hepatology, 60(6), 1235-1241 (2014-02-18)
Therapeutic management of liver fibrosis remains an unsolved clinical problem. Hepatic accumulation of extracellular matrix, mainly collagen, is mediated by the production of transforming growth factor-β1 (TGF-β1) in stellate cells. Pin1, a peptidyl-prolyl isomerase, plays an important pathophysiological role in
Garam Kim et al.
Molecular carcinogenesis, 54(6), 440-448 (2013-11-23)
Pin1, a conserved eukaryotic Peptidyl-prolyl cis/trans isomerase, has profound effects on numerous key-signaling molecules, and its deregulation contributes to disease, particularly cancer. Although Pin1-mediated prolyl isomerization is an essential and novel regulatory mechanism for protein phosphorylation, little is known about
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service