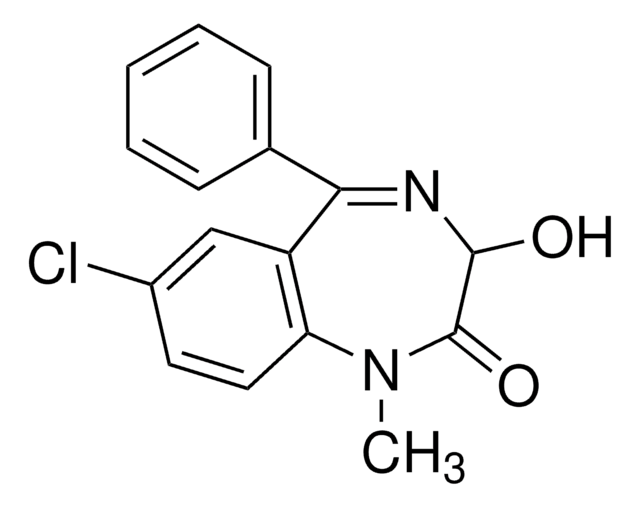
D-907
Diazepam solution
1.0 mg/mL in methanol, ampule of 1 mL, certified reference material, Cerilliant®
About This Item
Recommended Products
grade
certified reference material
Quality Level
form
liquid
feature
Snap-N-Spike®/Snap-N-Shoot®
packaging
ampule of 1 mL
manufacturer/tradename
Cerilliant®
drug control
Narcotic Licence Schedule B (Switzerland); psicótropo (Spain); Decreto Lei 15/93: Tabela IV (Portugal)
concentration
1.0 mg/mL in methanol
technique(s)
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
application(s)
clinical testing
format
single component solution
storage temp.
-10 to -25°C
SMILES string
CN1C(=O)CN=C(c2ccccc2)c3cc(Cl)ccc13
InChI
1S/C16H13ClN2O/c1-19-14-8-7-12(17)9-13(14)16(18-10-15(19)20)11-5-3-2-4-6-11/h2-9H,10H2,1H3
InChI key
AAOVKJBEBIDNHE-UHFFFAOYSA-N
Gene Information
human ... GABRA1(2554) , GABRA2(2555) , GABRA3(2556) , GABRA4(2557) , GABRA5(2558) , GABRA6(2559) , GABRB1(2560) , GABRB2(2561) , GABRB3(2562) , GABRD(2563) , GABRE(2564) , GABRG1(2565) , GABRG2(2566) , GABRG3(2567) , GABRP(2568) , GABRQ(55879)
Looking for similar products? Visit Product Comparison Guide
General description
Diazepam is an anxiolytic benzodiazepine drug, commonly used to treat anxiety disorders, muscle spasms, and acute seizures among others.
Application
- Electrochemical determination of diazepam in serum, urine, and tablet formulations by a modified electrode based on fullerene-functionalized carbon nanotubes and ionic liquid (C60-CNTs/IL)
- Differential voltametric analysis of diazepam in its tablet formulations and human urine samples using a bismuth-modified pre-treated pencil graphite electrode (BiPPGE)
- Simultaneous quantitative determination of diazepam and flunitrazepam in alcoholic beverage samples by Direct-electron ionization-liquid chromatography-tandem mass spectrometry (EI-LC-MS/MS) method
- Dispersive nanomaterial-ultrasound assisted-microextraction (DNUM) of diazepam and chlordiazepoxide from urine and plasma samples for their quantification by high-performance liquid chromatography -UV (HPLC-UV) detection
- Multi-residue analysis of diazepam and its metabolites in human oral fluid samples by their solid-phase extraction followed by LC-MS/MS method-based determination
Features and Benefits
- Fully characterized under ISO/IEC 17025 and ISO 17034 accreditation
- Accompanied with a comprehensive Certificate of Analysis (CoA) with data on stability, homogeneity, accuracy of concentration, uncertainty, and traceability
- Rigorously tested through real-time stability studies to ensure accuracy and shelf life
- Gravimetrically prepared using qualified precision balances to ensure minimal uncertainty
- Flame sealed under argon into ampoules for long-term shelf life
- Offered in a convenient, DEA-exempt format to improve laboratory efficiency
Legal Information
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - STOT SE 1
Target Organs
Eyes,Central nervous system
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
49.5 °F - closed cup
Flash Point(C)
9.7 °C - closed cup
Choose from one of the most recent versions:
Certificates of Analysis (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documents section.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
This study compares the use of SPME LC Tips to dried blood spots (DBS) in terms of extraction efficiency and detection limits of target analytes from whole blood.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| D-907-1ML | 4061833557419 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service