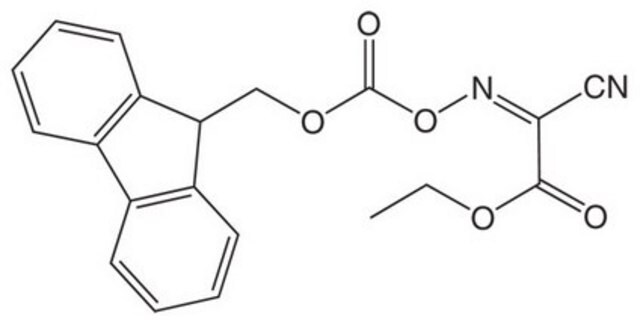
712191
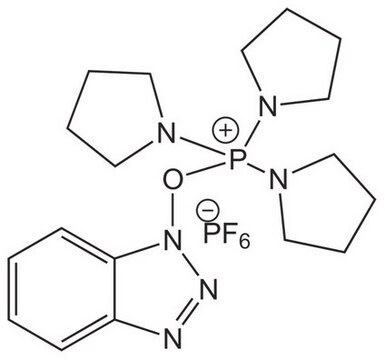
COMU®
≥96.5% (TLC), for peptide synthesis
Synonym(s):
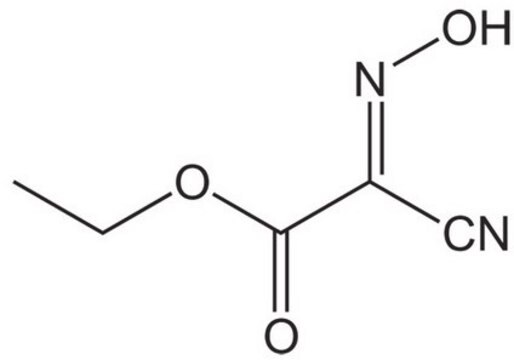
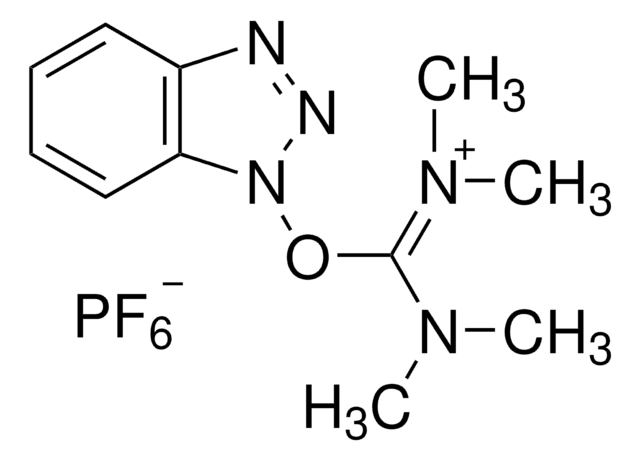
(1-Cyano-2-ethoxy-2-oxoethylidenaminooxy)dimethylamino-morpholino-carbenium hexafluorophosphate
About This Item
Recommended Products
product name
COMU®, 97%
Quality Level
Assay
≥96.5% (TLC)
96.5-103.5% (T)
97%
form
crystals
reaction suitability
reaction type: Coupling Reactions
greener alternative product characteristics
Less Hazardous Chemical Syntheses
Inherently Safer Chemistry for Accident Prevention
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
application(s)
peptide synthesis
greener alternative category
, Aligned
storage temp.
2-8°C
SMILES string
F[P-](F)(F)(F)(F)F.CCOC(=O)C(=NO\C(N1CCOCC1)=[N+](/C)C)C#N
InChI
1S/C12H19N4O4.F6P/c1-4-19-11(17)10(9-13)14-20-12(15(2)3)16-5-7-18-8-6-16;1-7(2,3,4,5)6/h4-8H2,1-3H3;/q+1;-1/b14-10-;
InChI key
GPDHNZNLPKYHCN-DZOOLQPHSA-N
General description
Application
Advantages
- Equal or even superior performance to HATU
- Non-explosive (does not contain benzotriazole moiety)
- Suitable for solution phase & solid phase peptide synthesis
- Utmost retention of configuration – low to non-existent racemization
- High solubility and stability in typical solvents
- Visual or colorimetric reaction monitoring possible
- Easy removal of water-soluble by-products
Legal Information
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
COMU is a non-explosive coupling agent suitable for solution phase & solid phase peptide synthesis. Its activity meets or exceeds that of HATU and its water-soluble by-product are easily removed.
Amide bonds are ubiquitous in both nature and industrial applications. They are vital to the structure and function of biological macromolecules and polymers. The importance of this functionality has resulted in numerous approaches to its formation, ranging from stoichiometric activation of carboxylic acids to more recent advances in catalytic amide bond formation.
Protocols
Amide Coupling in a Box
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![COMU 1-[(1-(Cyano-2-ethoxy-2-oxoethylideneaminooxy) dimethylaminomorpholino)] uronium hexafluorophosphate Novabiochem®](/deepweb/assets/sigmaaldrich/product/images/237/337/13566c06-8931-4cc2-8621-c8742a392cd6/640/13566c06-8931-4cc2-8621-c8742a392cd6.jpg)