12804
HBTU
≥98.0% (T), for peptide synthesis
Synonym(s):
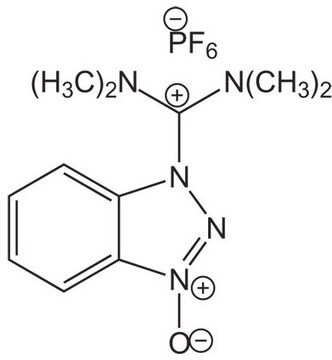
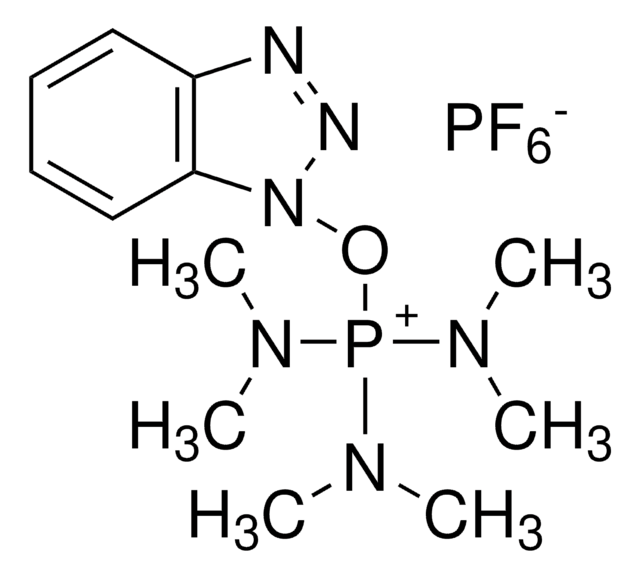
N,N,N′,N′-Tetramethyl-O-(1H-benzotriazol-1-yl)uronium hexafluorophosphate, O-(Benzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate
About This Item
Recommended Products
product name
HBTU, ≥98.0% (T)
Quality Level
Assay
≥98.0% (T)
form
solid
reaction suitability
reaction type: Coupling Reactions
mp
200 °C (dec.) (lit.)
solubility
acetonitrile: 0.1 g/mL, clear
application(s)
peptide synthesis
storage temp.
2-8°C
SMILES string
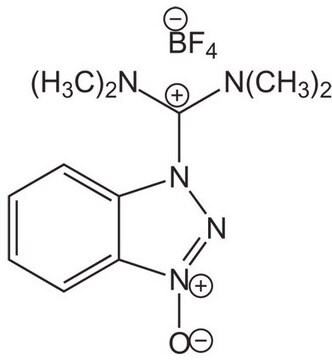
F[P-](F)(F)(F)(F)F.CN(C)C(\On1nnc2ccccc12)=[N+](/C)C
InChI
1S/C11H16N5O.F6P/c1-14(2)11(15(3)4)17-16-10-8-6-5-7-9(10)12-13-16;1-7(2,3,4,5)6/h5-8H,1-4H3;/q+1;-1
InChI key
UQYZFNUUOSSNKT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Sens. 1A
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
The special need of SPPS for rapid and highly efficient coupling reagents led to the development of several new reagents starting from BOP (Benzotriazol-1-yloxytris(dimethylamino)phosphonium hexafluorophosphate).
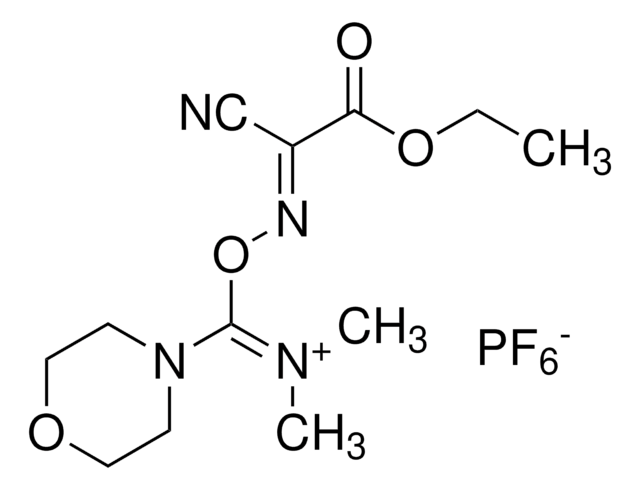
COMU is a non-explosive coupling agent suitable for solution phase & solid phase peptide synthesis. Its activity meets or exceeds that of HATU and its water-soluble by-product are easily removed.
Amide bonds are ubiquitous in both nature and industrial applications. They are vital to the structure and function of biological macromolecules and polymers. The importance of this functionality has resulted in numerous approaches to its formation, ranging from stoichiometric activation of carboxylic acids to more recent advances in catalytic amide bond formation.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service