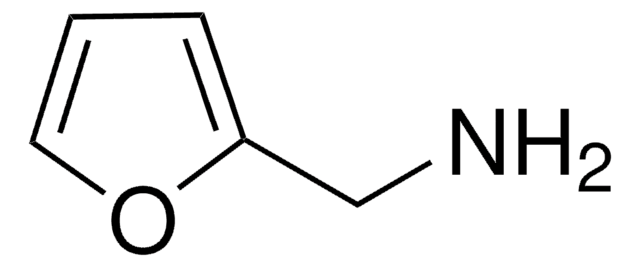
48190
(±)-1-(2-Furyl)ethanol
≥99.0% (GC)
Synonym(s):
(±)-α-Methylfuran-2-methanol, (±)-2-Furyl methyl carbinol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H8O2
CAS Number:
Molecular Weight:
112.13
Beilstein:
107814
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥99.0% (GC)
contains
~0.05% hydroquinone as stabilizer
refractive index
n20/D 1.479
bp
167-170 °C
density
1.078 g/mL at 20 °C (lit.)
storage temp.
2-8°C
SMILES string
CC(O)c1ccco1
InChI
1S/C6H8O2/c1-5(7)6-3-2-4-8-6/h2-5,7H,1H3
InChI key
UABXUIWIFUZYQK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
(±)-1-(2-Furyl)ethanol [(±)-2-Furyl methyl carbinol] is a furan derivative. The synthesis of 4-hydroxy-2-methylcyclopent-2-en-1-one from 2-furyl methyl carbinol has been reported.
Application
(±)-1-(2-Furyl)ethanol (Racemic 1-(2-furyl)ethanol) may be used in the synthesis of 1-acetoxy-1-[2-furyl]ethan.
Caution
may discolor to brown on storage
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A Ghanem et al.
Chirality, 13(2), 118-123 (2001-02-15)
Asymmetric acetylation of racemic 1-(2-furyl)ethanol with the innocuous acyl donor isopropenyl acetate catalyzed by lipases in organic media afforded the chiral alcohol and acetate in high enantiomeric excess (up to 99%). The effect of molecular sieves as well as organic
An Enantioselective Total Synthetic Approach to (+)-Heptemerone G and (+)-Guanacastepene A from 2-Furyl Methyl Carbinol.
Michalak K and Wicha J.
Synlett, 24, 1387-1390 (2013)
Jordan Lopez et al.
Journal of agricultural and food chemistry, 67(41), 11444-11453 (2019-10-09)
Innovative approaches to develop flavors with high sensory appeal are critical in encouraging increased consumer preference and adoption of low sodium foods. Gas chromatography-olfactometry, coupled with stable isotope dilution assays and sensory experiments, led to the identification of the odorants
Zhen Zeng et al.
Food chemistry, 188, 591-595 (2015-06-05)
The formation of 2-vinylfuran from the corresponding 4-oxo-2-hexenal (OHE, a lipid oxidation product) under the catalysis of amino acid were studied. The effects of amino acids, reaction temperature, reaction time, water content, pH, metallic ions and some food additives on
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service