Aerobic Alcohol Oxidation Solutions
Introduction
Alcohol oxidation is one of the most frequently performed oxidation reactions in organic chemistry. The aldehyde and ketone products of alcohol oxidation are useful intermediates en route to complex molecules. Molecular oxygen is an ideal oxidant, but aerobic oxidation reactions are seldom used on a bench scale due to limited synthetic scope, practicality, and safety concerns. The Stahl Aerobic Oxidation Solutions (TEMPO: 796549 and ABNO: 796557), when used with copper source[Cu(MeCN04]OTf (Product No. 685038), operate with ambient air as the source of oxidant and offer a safe and practical way to achieve high reactivity and selectivity in the aerobic oxidation of alcohols to aldehydes and ketones.
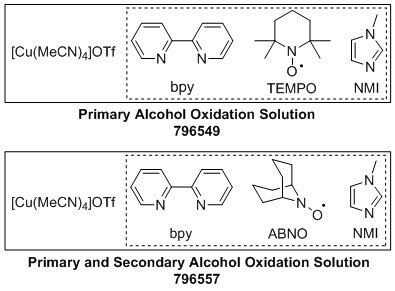
Advantages
- Easy reaction set-up (just add substrate and copper catalyst) and work-up procedures
- Most reactions proceed at room temperature open to the air
- Reaction completion is indicated by change in color from red/brown to green or blue
- TEMPO offers chemoselective oxidation of primary alcohols
- ABNO offers rapid oxidation of both primary and secondary alcohols
- Broad substrate scope and high functional group tolerance
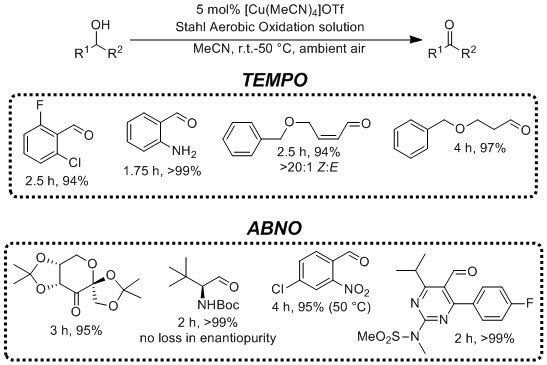
Representative Applications
References
Para seguir leyendo, inicie sesión o cree una cuenta.
¿No tiene una cuenta?