P8124
Pyoverdines
from Pseudomonas fluorescens, >90% (HPLC)
Sinónimos:
Pyoverdine Detection, Pyoverdine Protein, Siderophore Protein
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
UNSPSC Code:
12352200
NACRES:
NA.32
Productos recomendados
biological source
Pseudomonas fluorescens
Quality Level
assay
>90% (HPLC)
form
powder
solubility
H2O: ~10 mg/mL
shipped in
wet ice
storage temp.
−20°C
¿Está buscando productos similares? Visita Guía de comparación de productos
Application
Pyoverdines from Pseudomonas fluorescens has been used :
- in tryptophan fluorescence quenching to determine its binding to neutrophil-gelatinase-associated lipocalin (NGAL)
- to detect pyoverdine diffusion surrounding siderophore-conjugated monobactam (MB-1)-resistant colonies in chelexed, dialyzed Mueller-Hinton broth (CDMHB)
- for synchronous fluorescence spectra of purified pyoverdine
Biochem/physiol Actions
Pyoverdines help in promoting plant growth by chelating iron and rendering it unavailable for plant pathogens. The ferri-pyoverdine complex acts as a signaling molecule inducing the production of secreted virulence factors in Pseudomonas aeruginosa.
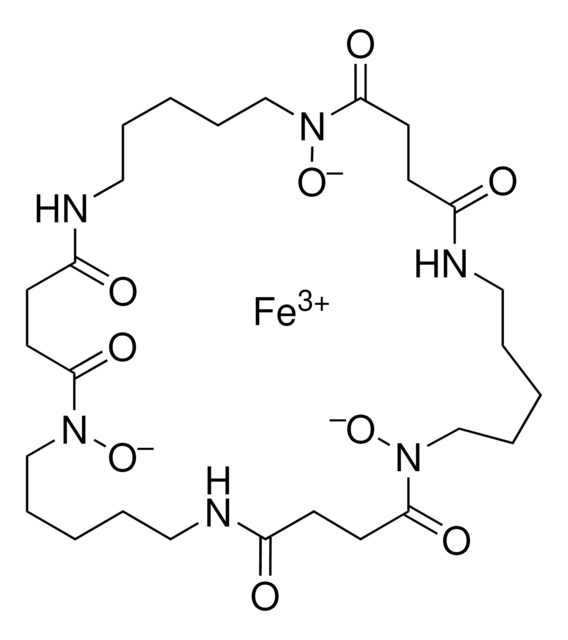
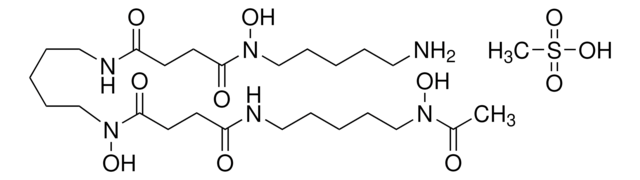
Pyoverdines, also called pseuobactins and pyoverdins, are fluorescent siderophores that have high affinity for iron (1032 M-1), and are synthesized by fluorescent pseudomonads under iron-deficient growth conditions. Pyoverdines were shown to prevent iron toxicity produced by iron overload in hepatocyte cultures and effectively scavenges the hydroxyl and peroxyl radicals. Pyoverdines are effective in acquiring iron from transferrin and lactoferrin. Pyoverdines are also involved in the suppression of pythium-induced damping-off of tomato and promotion of growth in some higher plants.
Pyoverdines, also called pseuobactins and pyoverdins, are fluorescent siderophores that have high affinity for iron, and are synthesized by fluorescent pseudomonads under iron-deficient growth conditions.
Physical form
Supplied as a mixture containing mainly the succinic acid (MW=1161), 2-hydroxy glutaramide (MW=1190), and succinamide (MW=1160) forms of pyoverdines.
related product
Referencia del producto
Descripción
Precios
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Antioxidant and free radical scavenging activities of the iron chelators pyoverdin and hydroxypyrid-4-ones in iron-loaded hepatocyte cultures: comparison of their mechanism of protection with that of desferrioxamine.
Morel I
Free Radical Biology & Medicine, 13(5), 499-508 (1992)
Adaptation-based resistance to siderophore-conjugated antibacterial agents by Pseudomonas aeruginosa.
Tomaras AP
Antimicrobial Agents and Chemotherapy, 57(9), 4197-4207 (2013)
Purification of Pyoverdines of Pseudomonas fluorescens 2-79 by Copper-Chelate Chromatography.
Xiao R and Kisaalita WS
Applied and Environmental Microbiology, 61(11), 3769-3774 (1995)
Biological activity of secondary metabolites produced by a strain of Pseudomonas fluorescens.
Boruah HP and Kumar BS
Folia Microbiologica, 47(4), 359-363 (2002)
Correction: Multicolor Whole-Cell Bacterial Sensing Using a Synchronous Fluorescence Spectroscopy-Based Approach.
PLoS ONE (2015)
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico