P0122
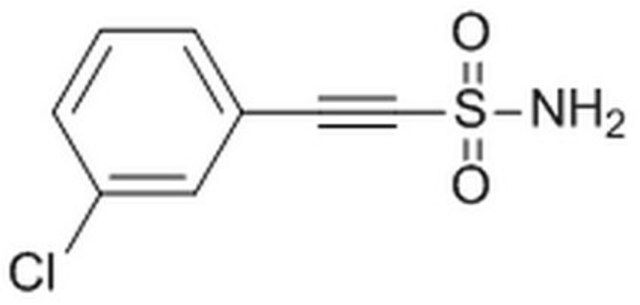
Pifithrin-μ
≥97% (HPLC), solid
Sinónimos:
2-Phenyl-ethynesulfoanide, PFT-μ
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C8H7NO2S
Número de CAS:
Peso molecular:
181.21
MDL number:
UNSPSC Code:
12352200
PubChem Substance ID:
NACRES:
NA.77
Productos recomendados
Quality Level
assay
≥97% (HPLC)
form
solid
storage condition
desiccated
solubility
DMSO: soluble >10 mg/mL, clear
H2O: insoluble
storage temp.
2-8°C
SMILES string
NS(=O)(=O)C#Cc1ccccc1
InChI
1S/C8H7NO2S/c9-12(10,11)7-6-8-4-2-1-3-5-8/h1-5H,(H2,9,10,11)
InChI key
ZZUZYEMRHCMVTB-UHFFFAOYSA-N
Application
Pifithrin-μ has been used:
- to treat microglial cell line to analyse its neuroprotective effect on M1-like and M2-like phenotype
- as heat shock protein (HSP)-70 inhibitor, to treat transfected Marc-145 cells
- to inhibit heat shock cognate 70 (Hsc70) to elucidate heat shock chaperones mouse embryonic stem cells
Biochem/physiol Actions
Pifithrin-μ is an inhibitor of p53 binding and anti-apoptotic, which directly inhibits p53 binding to mitochondria as well as to Bcl-xL and Bcl-2 proteins.
Pifithrin-μ is an inhibitor of p53 binding and anti-apoptotic, which directly inhibits p53 binding to mitochondria as well as to Bcl-xL and Bcl-2 proteins. PFTμ rescues cells from lethal γ-irradiation-induced cell death. Because pifithrin-μ shuts down only the p53-mitochondrial pathway without affecting the transcriptional functions of p53, it is superior to pifithrin-α.
Pifithrin-μ(PFT-μ) has neuroprotective capabilities against cell death in a preclinical model of hypoxia-ischemia (HI)-induced neonatal encephalopathy. It inhibits nuclear factor-ΙB (NF-ΙB) pathway by inhibiting the interaction of molecular chaperone heat shock protein (HSP)-70 with its substrates.
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Julia I-Ju Leu et al.
ACS chemical biology, 9(11), 2508-2516 (2014-08-26)
The stress-inducible mammalian heat shock protein 70 (HSP70) and its bacterial orthologue DnaK are highly conserved nucleotide binding molecular chaperones. They represent critical regulators of cellular proteostasis, especially during conditions of enhanced stress. Cancer cells rely on HSP70 for survival
J I-Ju Leu et al.
Molecular cell, 36(1), 15-27 (2009-10-13)
The multifunctional, stress-inducible molecular chaperone HSP70 has important roles in aiding protein folding and maintaining protein homeostasis. HSP70 expression is elevated in many cancers, contributing to tumor cell survival and resistance to therapy. We have determined that a small molecule
Determination of the interactome of non-structural protein12 from highly pathogenic porcine reproductive and respiratory syndrome virus with host cellular proteins using high throughput proteomics and identification of HSP70 as a cellular factor for virus replication
Dong S, et al.
Journal of Proteomics, 146, 58-69 (2016)
Wonkyoung Cho et al.
Molecular and cellular biology, 39(9) (2019-02-13)
Delineating the mechanisms that drive hepatic injury and hepatocellular carcinoma (HCC) progression is critical for development of novel treatments for recurrent and advanced HCC but also for the development of diagnostic and preventive strategies. Heat shock protein 70 (HSP70) acts
Fernando Mérida et al.
International journal of nanomedicine, 15, 419-432 (2020-02-06)
Magnetic Fluid Hyperthermia (MFH) is a promising adjuvant for chemotherapy, potentiating the action of anticancer agents. However, drug delivery to cancer cells must be optimized to improve the overall therapeutic effect of drug/MFH combination treatments. The aim of this work
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico