K1876
Kanamycin disulfate salt from Streptomyces kanamyceticus
aminoglycoside antibiotic
Sinónimos:
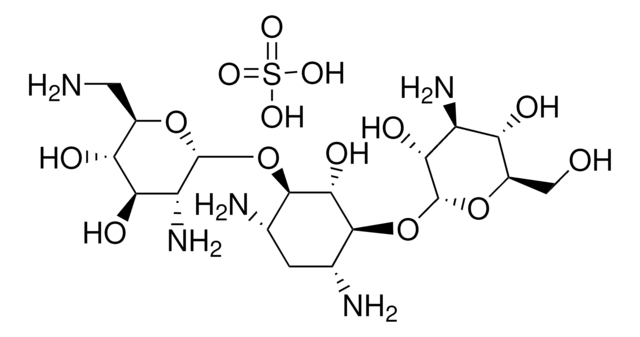
Kanamycin Disulfate Salt, Kanamycin disulfate, O-3-amino-3-deoxy-alpha-D-glucopyranosyl-(1->6)-O-(6-amino-6-deoxy-alpha-D-glucopyranosyl-(1->4))-2-deoxy-D-Streptamine sulfate (1:2) (salt), Kanamycin A, Kanamycin acid sulfate
About This Item
Productos recomendados
biological source
Streptomyces kanamyceticus
Quality Level
form
powder
potency
~650 μg per mg
impurities
≤4% Kanamycin B
color
white to off-white
solubility
H2O: 10 mg/mL
antibiotic activity spectrum
Gram-negative bacteria
Gram-positive bacteria
mycobacteria
mycoplasma
mode of action
protein synthesis | interferes
SMILES string
OS(O)(=O)=O.OS(O)(=O)=O.NC[C@H]1O[C@H](O[C@@H]2[C@@H](N)C[C@@H](N)[C@H](O[C@H]3O[C@H](CO)[C@@H](O)[C@H](N)[C@H]3O)[C@H]2O)[C@H](O)[C@@H](O)[C@@H]1O
InChI
1S/C18H36N4O11.2H2O4S/c19-2-6-10(25)12(27)13(28)18(30-6)33-16-5(21)1-4(20)15(14(16)29)32-17-11(26)8(22)9(24)7(3-23)31-17;2*1-5(2,3)4/h4-18,23-29H,1-3,19-22H2;2*(H2,1,2,3,4)/t4-,5+,6-,7-,8+,9-,10-,11-,12+,13-,14-,15+,16-,17-,18-;;/m1../s1
InChI key
OGTKIXVMLDAMNU-KNQICTBBSA-N
General description
Application
Biochem/physiol Actions
Mode of Resistance: Aminoglycoside-modifying enzymes (including acetyltransferase, phosphotransferase, nucleotidyltransferase) can alter this antibiotic, preventing its interaction with ribosomes.
Antimicrobial Spectrum: Kanamycin sulfate is effective against gram-negative and gram-postiive bacteria, and mycoplasma.
Caution
Preparation Note
signalword
Danger
hcodes
Hazard Classifications
Repr. 1B
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Protein synthesis is a complex, multi-step process involving many enzymes as well as conformational alignment. However, the majority of antibiotics that block bacterial protein synthesis interfere with the processes at the 30S subunit or 50S subunit of the 70S bacterial ribosome.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico