G6752
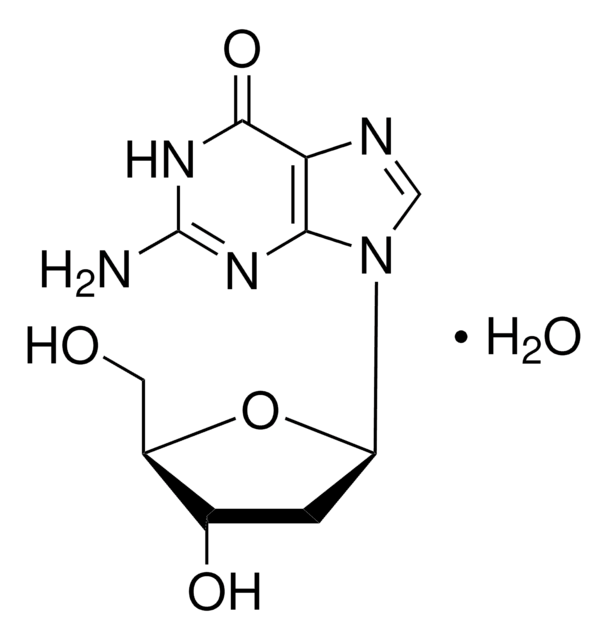
Guanosine
≥98%
Sinónimos:
9-(β-D-Ribofuranosyl)guanine, Guanine-9-β-D-ribofuranoside
About This Item
Productos recomendados
biological source
microbial
Quality Level
100
200
assay
≥98%
form
powder
mp
250 °C (dec.) (lit.)
solubility
formic acid:water (1:1): 50 mg/mL, clear to very slightly hazy, colorless to faintly yellow
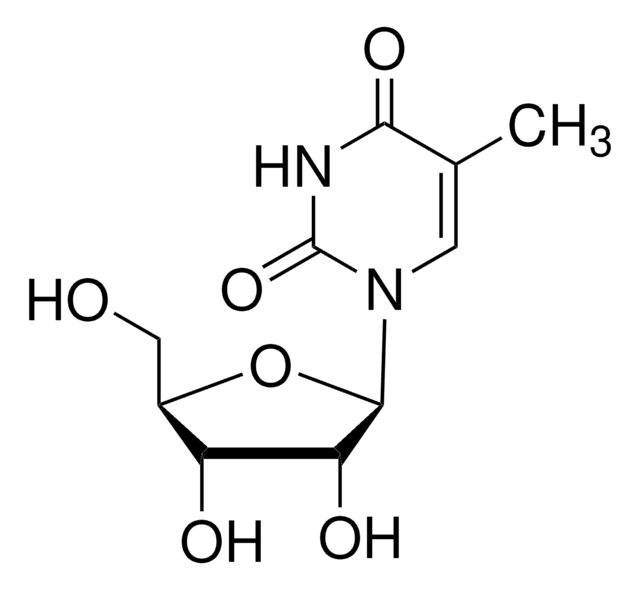
SMILES string
[H]O[H].NC1=Nc2c(ncn2[C@@H]3O[C@H](CO)[C@@H](O)[C@H]3O)C(=O)N1
InChI
1S/C10H13N5O5/c11-10-13-7-4(8(19)14-10)12-2-15(7)9-6(18)5(17)3(1-16)20-9/h2-3,5-6,9,16-18H,1H2,(H3,11,13,14,19)/t3-,5-,6-,9-/m1/s1
InChI key
NYHBQMYGNKIUIF-UUOKFMHZSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
General description
Application
- as a reference standard for the analysis of glucosinolates by high-performance liquid chromatography with diode-array detection and electrospray ionization tandem mass spectrometry (HPLC-DAD-ESI/MS)
- as a component of Mouse Embryonic Fibroblasts (MEFs) culture
- as a standard for the detection of residual RNA contaminant in oil palm plant genome samples by HPLC
Biochem/physiol Actions
Storage Class
11 - Combustible Solids
wgk_germany
WGK 2
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico