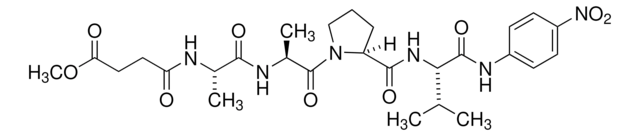
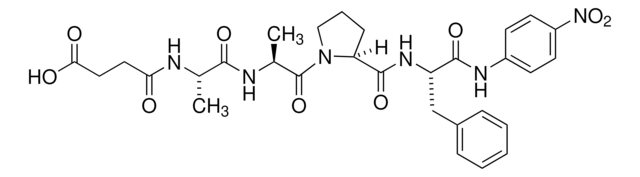
E0258
Elastase from porcine pancreas
Type IV, Protein 50-90 %, lyophilized powder, ≥4.0 units/mg protein (biuret)
Sinónimos:
Elastase from hog pancreas, Pancreatopeptidase E
About This Item
Productos recomendados
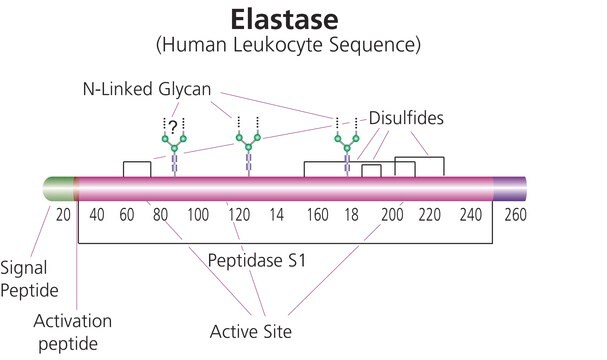
biological source
Porcine pancreas
type
Type IV
form
lyophilized powder
specific activity
≥4.0 units/mg protein (biuret)
composition
Protein, 50-90%
foreign activity
trypsin ≤50 BAEE units/mg protein
storage temp.
−20°C
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Application
- to treat vero cells to study its effects on syncytium formation
- as a positive control in protease assays
- as a component in RPMI 1640 to isolate human aortic smooth muscle cells (HASMCs) from the aortic tissue
Biochem/physiol Actions
Packaging
Unit Definition
Physical form
Preparation Note
Application
inhibitor
signalword
Danger
hcodes
Hazard Classifications
Eye Irrit. 2 - Resp. Sens. 1 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Chromatograms
application for HPLCNuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico

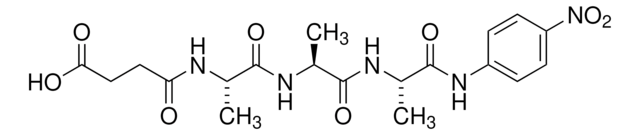




![N-[3-(2-Furyl)acryloyl]-Leu-Gly-Pro-Ala](/deepweb/assets/sigmaaldrich/product/structures/805/876/96b5fb57-71c8-4c6b-b5d2-fafe7374cd85/640/96b5fb57-71c8-4c6b-b5d2-fafe7374cd85.png)