D5782
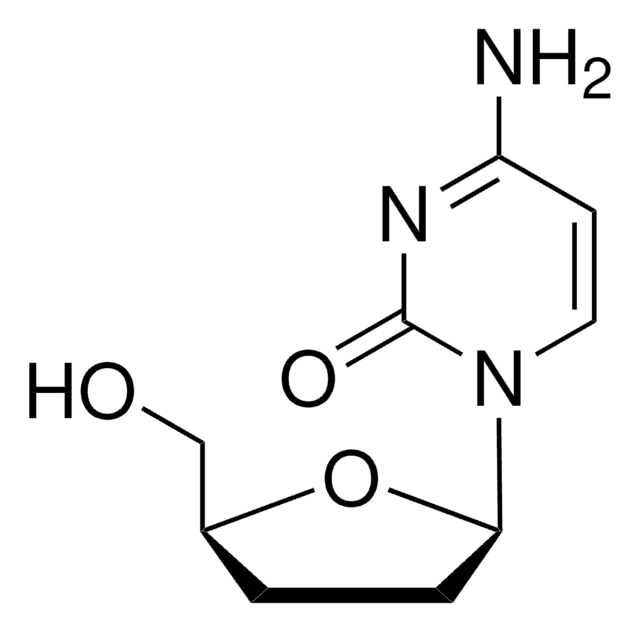
2′,3′-Dideoxycytidine
≥98% (HPLC)
Sinónimos:
ddC
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C9H13N3O3
Número de CAS:
Peso molecular:
211.22
Beilstein/REAXYS Number:
654956
MDL number:
UNSPSC Code:
41106305
PubChem Substance ID:
NACRES:
NA.52
Productos recomendados
biological source
synthetic (organic)
assay
≥98% (HPLC)
form
powder
color
colorless
mp
217-218 °C (lit.)
solubility
water: 50 mg/mL, clear, colorless to faintly yellow
storage temp.
−20°C
SMILES string
NC1=NC(=O)N(C=C1)[C@H]2CC[C@@H](CO)O2
InChI
1S/C9H13N3O3/c10-7-3-4-12(9(14)11-7)8-2-1-6(5-13)15-8/h3-4,6,8,13H,1-2,5H2,(H2,10,11,14)/t6-,8+/m0/s1
InChI key
WREGKURFCTUGRC-POYBYMJQSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Application
2′,3′-Dideoxycytidine is used:
- as a DNA chain-terminating nucleotide for DNA sequencing methods based on the Sanger chain-termination method
- as a nucleoside reverse transcriptase inhibitor (NRTI) to study its effects on the development of mechanical allodynia in aging mice
- as a mitochondrial DNA (mtDNA) replication inhibitor to inhibit the activation of cGAS-STING pathway and study its effects on signaling protein-stimulator of interferon genes (STING), cyclic GMP-AMP synthase (cGAS), and phospho-interferon regulator factor 3 (p-IRF3) expression in mouse hippocampal and microglial cells
- as an NRTI inhibitor to study its effects on the drug induced-mitochondrial toxicity in Caenorhabditis elegans
Biochem/physiol Actions
2′,3′-Dideoxycytidine (ddC), is an ionic compound and a nucleoside analog. It acts as a nucleoside reverse transcriptase inhibitor and exhibits therapeutic effects against human immunodeficiency virus (HIV) infection. 2′,3′-Dideoxycytidine possesses anti-adenovirus activity and inhibits the adenovirus polymerase.
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Maja M Janas et al.
Nucleic acids research, 47(7), 3306-3320 (2019-03-02)
For oligonucleotide therapeutics, chemical modifications of the sugar-phosphate backbone are frequently used to confer drug-like properties. Because 2'-deoxy-2'-fluoro (2'-F) nucleotides are not known to occur naturally, their safety profile was assessed when used in revusiran and ALN-TTRSC02, two short interfering
Shuang-Xi Gu et al.
Bioorganic & medicinal chemistry, 19(17), 5117-5124 (2011-08-10)
A series of 26 diarylpyrimidines, characterized by the hydroxymethyl linker between the left wing benzene ring and the central pyrimidine, were synthesized and evaluated for in vitro anti-HIV activity. Most of the compounds exhibited moderate to excellent activities against wild-type
Chunju Fang et al.
Cellular & molecular immunology, 18(9), 2211-2223 (2020-05-14)
Exposure to ionizing radiation, a physical treatment that inactivates live tumor cells, has been extensively applied to enhance the antitumor responses induced by cancer cell vaccines in both animal research and human clinical trials. However, the mechanisms by which irradiated
Anindya Roy Chowdhury et al.
iScience, 23(8), 101370-101370 (2020-08-02)
This study shows that multiple modes of mitochondrial stress generated by partial mtDNA depletion or cytochrome c oxidase disruption cause ryanodine receptor channel (RyR) dysregulation, which instigates the release of Ca2+ in the cytoplasm of C2C12 myoblasts and HCT116 carcinoma
Didier Desmaële et al.
Journal of controlled release : official journal of the Controlled Release Society, 161(2), 609-618 (2011-08-16)
Squalene is a triterpene widely distributed in nature that is an intermediate in the cholesterol biosynthesis pathway. The remarkable dynamic folded conformation of squalene has been used to chemically conjugate this lipid with various therapeutic molecules to construct nanoassemblies of
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico