A9934
Aminopeptidase I from Streptomyces griseus
lyophilized powder, ≥200 units/mg protein
Sinónimos:
Leucine Aminopeptidase IV
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Productos recomendados
form
lyophilized powder
Quality Level
specific activity
≥200 units/mg protein
mol wt
21 kDa by gel filtration
33 kDa by SDS-PAGE
composition
Protein, 40-60% Lowry
storage temp.
−20°C
General description
Aminopeptidase I from Streptomyces griseus is a thermostable enzyme with Glu131 and Tyr246 as key active site residues.
Application
Aminopeptidase I from Streptomyces griseus has been used:
- to test the biochar exposure effect on the enzyme activity
- in circular dichroism (CD) spectroscopy studies
- as a positive control in p-nitroanilide degradation assay
Biochem/physiol Actions
Aminopeptidase I from S. griseus has a fairly broad specificity, being able to remove the N-terminal residue of most proteins, except where the penultimate residue is an imino acid. It contains two Zn2+ binding sites. Aminopeptidase I from S. griseus is inhibited by 1,10-phenanthroline and is activated six-fold by Ca2+, which also stabilizes it against heat inactivation. This monomeric zinc metalloprotein has an isoelectric point (pI) of 5.4.
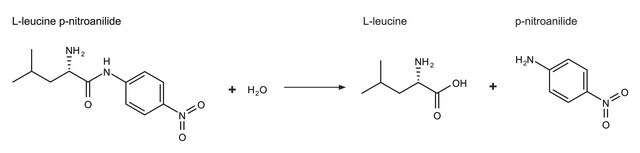
Aminopeptidase I may also be used as a reagent in the assay of endoprotease activities with a synthetic substrate in a two-stage assay. In the first stage, the endoprotease cleaves a peptide, such as Z-Y-X-Leu-p-nitroanilide, with the X, Y, and Z residues being chosen according to the specificity of the endoprotease.
Packaging
Package size based on protein content.
Unit Definition
One unit will hydrolyze 1.0 μmole of L-leucine-p-nitroanilide to L-leucine and p-nitroaniline per min at pH 8.0, 25 °C and 3.0 mM substrate concentration.
Physical form
Contains calcium acetate
Preparation Note
Reconstitute in 20 mM tricine, pH 8.0, with 0.05% bovine serum albumin. Dilute the enzyme with the reconstitution buffer to 0.15-0.3 U/mL for a working concentration. Solutions should be prepared fresh prior to use.
Other Notes
Endopeptidase contaminant: Not more than: 0.01 U/mg protein (as μmole tyrosine equivalent per min released from casein.)
signalword
Danger
hcodes
Hazard Classifications
Eye Irrit. 2 - Resp. Sens. 1 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
[Autophagy related genes in yeast, S. cerevisiae].
Yoshinori Ohsumi
Tanpakushitsu kakusan koso. Protein, nucleic acid, enzyme, 51(10 Suppl), 1453-1456 (2006-08-23)
Indig, F.E., et al.
Febs Letters, 225, 237-237 (1989)
Taras Y Nazarko et al.
Autophagy, 1(1), 37-45 (2006-07-29)
Yarrowia lipolytica was recently introduced as a new model organism to study peroxisome degradation in yeasts. Transfer of Y. lipolytica cells from oleate/ethylamine to glucose/ammonium chloride medium leads to selective macroautophagy of peroxisomes. To decipher the molecular mechanisms of macropexophagy
A Spungin et al.
European journal of biochemistry, 183(2), 471-477 (1989-08-01)
A heat-stable aminopeptidase with an N-terminal Ala-Pro-Asp-Ile-Pro-Leu sequence has been purified from Streptomyces griseus by heat treatment followed by gel-exclusion and anion-exchange chromatographic procedures. The enzyme is a monomeric zinc metalloenzyme showing an apparent molecular mass of 33 kDa by
Congcong He et al.
Molecular biology of the cell, 19(12), 5506-5516 (2008-10-03)
Autophagy is the degradation of a cell's own components within lysosomes (or the analogous yeast vacuole), and its malfunction contributes to a variety of human diseases. Atg9 is the sole integral membrane protein required in formation of the initial sequestering
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico