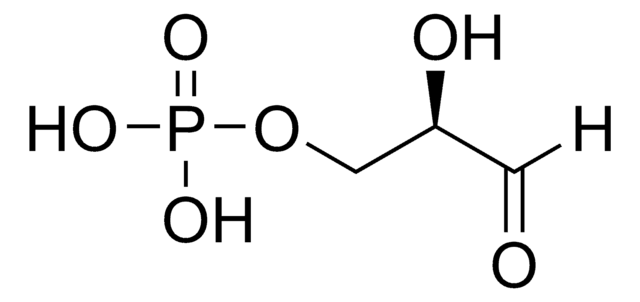
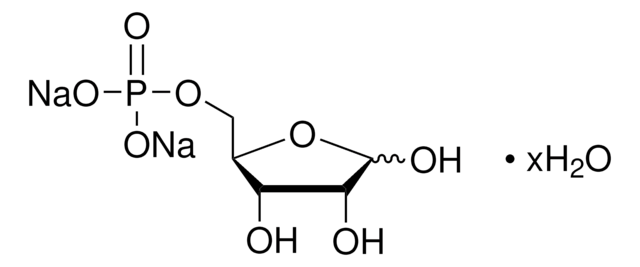
51269
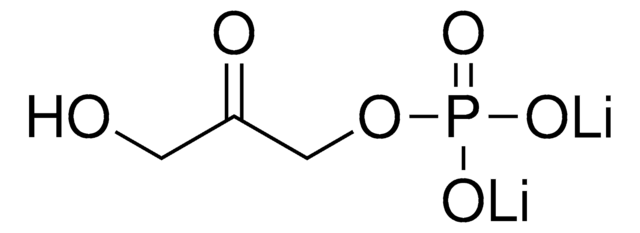
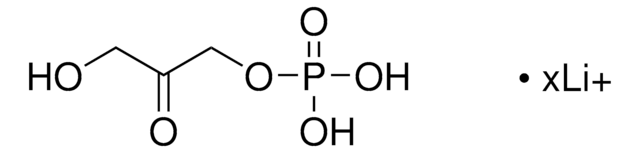
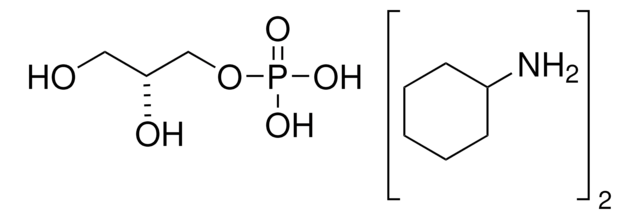
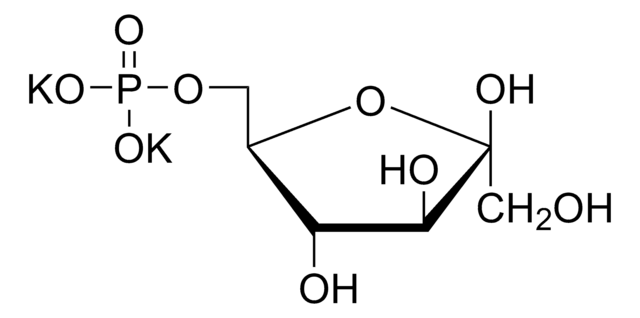
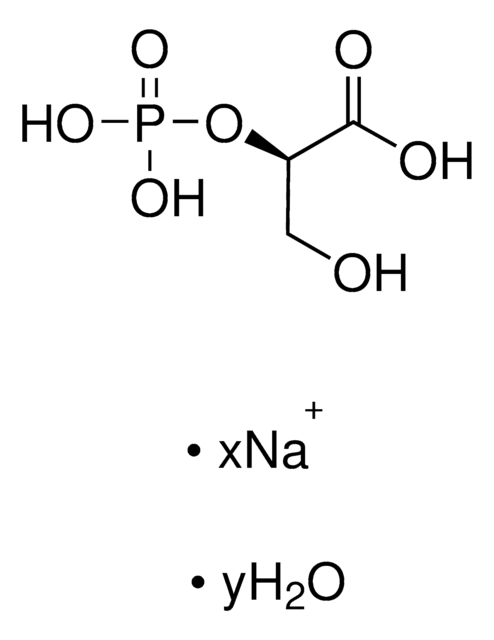
Dihydroxyacetone phosphate hemimagnesium salt hydrate
≥95% (TLC)
Sinónimos:
1,3-Dihydroxy-2-propanone 1-phosphate disodium salt, 1-Hydroxy-3-(phosphonooxy)-2-propanone disodium salt, DHAP Na2
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Productos recomendados
Quality Level
assay
≥95% (TLC)
storage temp.
−20°C
SMILES string
O.OCC(=O)COP(O)(=O)O[Mg]OP(O)(=O)OCC(=O)CO
InChI
1S/2C3H7O6P.Mg.H2O/c2*4-1-3(5)2-9-10(6,7)8;;/h2*4H,1-2H2,(H2,6,7,8);;1H2/q;;+2;/p-2
InChI key
XIBMKSGIYJARCI-UHFFFAOYSA-L
Application
Dihydroxyacetone phosphate hemimagnesium salt hydrate (DHAP Mg) is a magnesium salt of DHAP. DHAP may be used as the substrate to characterize enzymes such as fructose bisphosphate aldolase and triose phosphate isomerase.
Biochem/physiol Actions
Dihydroxyacetone phosphate (DHAP) is a metabolic intermediate involved in many pathways, including glycolysis, gluconeogenesis, glycerol metabolism, phosphatidic acid synthesis, fat metabolism, and the Calvin cycle.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Matthias Bujara et al.
Nature chemical biology, 7(5), 271-277 (2011-03-23)
Recruiting complex metabolic reaction networks for chemical synthesis has attracted considerable attention but frequently requires optimization of network composition and dynamics to reach sufficient productivity. As a design framework to predict optimal levels for all enzymes in the network is
Ferenc Orosz et al.
Biochimica et biophysica acta, 1792(12), 1168-1174 (2009-09-30)
The triosephosphate isomerase (TPI) functions at a metabolic cross-road ensuring the rapid equilibration of the triosephosphates produced by aldolase in glycolysis, which is interconnected to lipid metabolism, to glycerol-3-phosphate shuttle and to the pentose phosphate pathway. The enzyme is a
Alice Chan et al.
Angewandte Chemie (International ed. in English), 51(31), 7711-7714 (2012-06-21)
Stop for NadA! A [4Fe-4S] enzyme, NadA, catalyzes the formation of quinolinic acid in de novo nicotinamide adenine dinucleotide (NAD) biosynthesis. A structural analogue of an intermediate, 4,5-dithiohydroxyphthalic acid (DTHPA), has an in vivo NAD biosynthesis inhibiting activity in E. coli. The
Bjørn O Hald et al.
The FEBS journal, 279(23), 4410-4420 (2012-10-18)
Coherent glycolytic oscillations in Saccharomyces cerevisiae are a multicellular property induced by addition of glucose to a starved cell population of sufficient density. However, initiation of oscillations requires an additional perturbation, usually addition of cyanide. The fate of cyanide during
Michael Fischer et al.
Chemical communications (Cambridge, England), 47(23), 6647-6649 (2011-05-13)
An efficient gram scale synthesis of 3-fluoro-1-hydroxyacetone phosphate (FHAP) has been developed. As a close analog to dihydroxyacetone phosphate, FHAP was used as a novel donor substrate for rabbit muscle aldolase catalyzed reactions. The different binding affinities of the gem-diol
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico