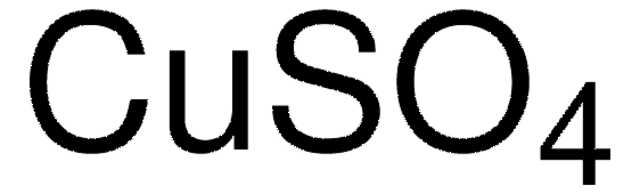12852
Copper(II) sulfate
puriss., meets analytical specification of Ph. Eur., BP, USP, anhydrous, 99-100.5% (based on anhydrous substance)
Sinónimos:
Cupric sulfate
About This Item
Productos recomendados
vapor pressure
7.3 mmHg ( 25 °C)
Quality Level
grade
puriss.
assay
99-100.5% (based on anhydrous substance)
form
solid
quality
meets analytical specification of Ph. Eur., BP, USP
impurities
residual solvents, complies
loss
≤1% loss on drying, 250 °C
pH
3.5-4.5 (20 °C, 50 g/L)
mp
200 °C (dec.) (lit.)
density
3.603 g/mL at 25 °C (lit.)
anion traces
chloride (Cl-): ≤100 mg/kg
cation traces
Ca: ≤50 mg/kg
Fe: ≤30 mg/kg
K: ≤100 mg/kg
Mg: ≤100 mg/kg
Na: ≤200 mg/kg
Ni: ≤50 mg/kg
Pb: ≤80 mg/kg
SMILES string
[Cu++].[O-]S([O-])(=O)=O
InChI
1S/Cu.H2O4S/c;1-5(2,3)4/h;(H2,1,2,3,4)/q+2;/p-2
InChI key
ARUVKPQLZAKDPS-UHFFFAOYSA-L
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
General description
It can find applications in agriculture as a nutrient for plants. It can also serve as a precursor in the production of foliar bactericides and fungicides.
Application
- As a catalyst for the acetylation of alcohols and phenols under solvent-free conditions.
- To compose the electrolyte for the electrodeposition of Cu-Zn-Sn precursors, required for the preparation of Cu2ZnSnS4 (CZTS) thin films.
- As a Lewis acid catalyst for the dehydration of alcohols.5
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Dam. 1
Storage Class
13 - Non Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico