D2757
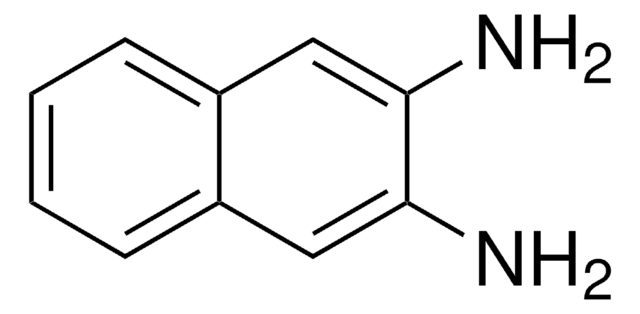
2,3-Diaminonaphthalene
≥95% purity (HPLC), powder
Sinónimos:
2,3-Naphthalenediamine, DAN
About This Item
Productos recomendados
Nombre del producto
2,3-Diaminonaphthalene, ≥95% (HPLC), powder
Quality Level
assay
≥95% (HPLC)
form
powder
technique(s)
titration: suitable
color
off-white to dark beige, to Dark Brown
mp
198-200 °C
solubility
pyridine: 50 mg/mL
application(s)
diagnostic assay manufacturing
hematology
histology
storage temp.
room temp
SMILES string
Nc1cc2ccccc2cc1N
InChI
1S/C10H10N2/c11-9-5-7-3-1-2-4-8(7)6-10(9)12/h1-6H,11-12H2
InChI key
XTBLDMQMUSHDEN-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
General description
Application
- 2,3-Diaminonaphthalene (DAN) has been used for the fluorometric measurement of nitrite/nitrate.
- DAN has been used to develop a solid phase extraction-multisyringe flow injection system to spectrophotometrically detect the presence of selenium.
- It has been employed in a study to develop a colorimetric assay for the detection of methylglyoxal.
- It has also been used in a study to develop a novel ratiometric fluorescent probe for the in-situ detection of alkaline phosphatase activity.
Biochem/physiol Actions
DAN also reacts with selenite to form 4,5-benzopiazselenol which is detectable via absorptiometry or fluorometry.
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Carc. 1A - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico