79245
(+)-Carvone
certified reference material, TraceCERT®, Manufactured by: Sigma-Aldrich Production GmbH, Switzerland
Sinónimos:
(+)-p-Mentha-6,8-diene 2-one, (S)-5-Isopropenyl-2-methyl-2-cyclohexenone
About This Item
Productos recomendados
grade
certified reference material
TraceCERT®
Quality Level
manufacturer/tradename
Manufactured by: Sigma-Aldrich Production GmbH, Switzerland
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
bp
228-230 °C (lit.)
density
0.960 g/mL at 20 °C (lit.)
application(s)
cleaning products
cosmetics
flavors and fragrances
food and beverages
personal care
format
neat
storage temp.
−20°C
SMILES string
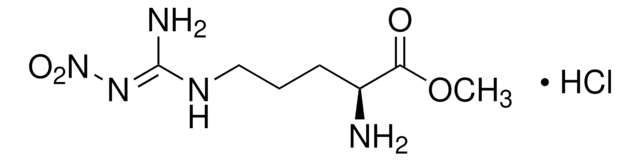
CC(=C)[C@H]1CC=C(C)C(=O)C1
InChI
1S/C10H14O/c1-7(2)9-5-4-8(3)10(11)6-9/h4,9H,1,5-6H2,2-3H3/t9-/m0/s1
InChI key
ULDHMXUKGWMISQ-VIFPVBQESA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Certified content by quantitative NMR incl. uncertainty and expiry date are given on the certificate.
Download your certificate at: http://www.sigma-aldrich.com.
Application
Packaging
Other Notes
Legal Information
signalword
Warning
hcodes
Hazard Classifications
Skin Sens. 1A
Storage Class
10 - Combustible liquids
wgk_germany
WGK 1
flash_point_f
204.1 °F - closed cup
flash_point_c
95.6 °C - closed cup
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico