517127
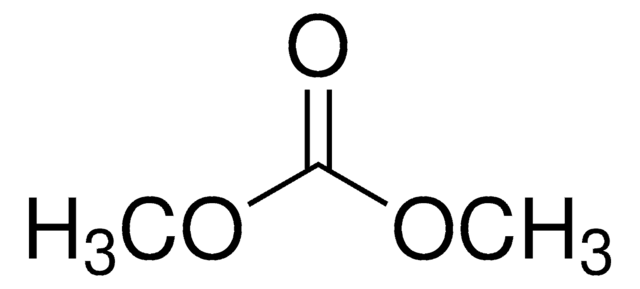
Dimethyl carbonate
anhydrous, ≥99%
Sinónimos:
DMC, Carbonic acid dimethyl ester
About This Item
Productos recomendados
grade
anhydrous
Quality Level
vapor density
3.1 (vs air)
vapor pressure
18 mmHg ( 21.1 °C)
assay
≥99%
form
liquid
expl. lim.
4.22-12.87 % (lit.)
greener alternative product characteristics
Less Hazardous Chemical Syntheses
Safer Solvents and Auxiliaries
Design for Degradation
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
impurities
<0.002% water
<0.005% water (100mL pkg)
color
APHA: <50
refractive index
n20/D 1.368 (lit.)
bp
90 °C (lit.)
mp
2-4 °C (lit.)
density
1.069 g/mL at 25 °C (lit.)
greener alternative category
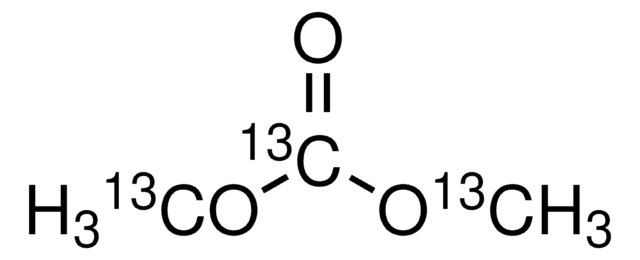
SMILES string
O=C(OC)OC
InChI
1S/C3H6O3/c1-5-3(4)6-2/h1-2H3
InChI key
IEJIGPNLZYLLBP-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
We are committed to bringing you Greener Alternative Products, which adhere to one or more of The 12 Principles of Greener Chemistry. This product is a Greener alternative to conventional solvents and chemicals. Click here for more information.
Application
Features and Benefits
signalword
Danger
hcodes
Hazard Classifications
Flam. Liq. 2
Storage Class
3 - Flammable liquids
wgk_germany
WGK 1
flash_point_f
60.8 °F - closed cup
flash_point_c
16 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
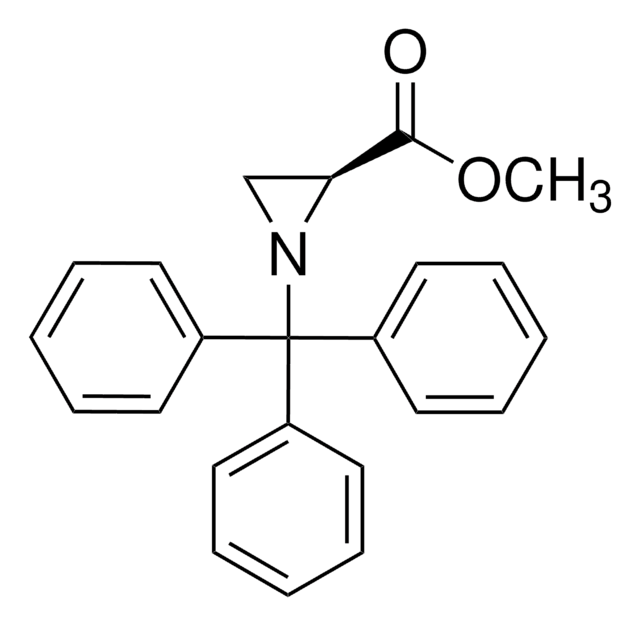
Amide bonds are ubiquitous in both nature and industrial applications. They are vital to the structure and function of biological macromolecules and polymers. The importance of this functionality has resulted in numerous approaches to its formation, ranging from stoichiometric activation of carboxylic acids to more recent advances in catalytic amide bond formation.
The critical technical challenges associated with the commercialization of electric vehicle batteries include cost, performance, abuse tolerance, and lifespan.
Contenido relacionado
Why should you have to choose between solvents that are ecological and those that are reliable? Enjoy both at once with our biorenewable and greener solutions. Cyrene™ solvent is a new dipolar aprotic alternative to common REACH restricted solvents, such as N methyl-2-pyrrolidone (NMP) and Dimethylformamide (DMF).
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico