45656
Rotenone
PESTANAL®, analytical standard
About This Item
Productos recomendados
grade
analytical standard
Quality Level
product line
PESTANAL®
shelf life
limited shelf life, expiry date on the label
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
bp
210-220 °C/0.5 mmHg (lit.)
mp
159-164 °C (lit.)
application(s)
agriculture
environmental
format
neat
SMILES string
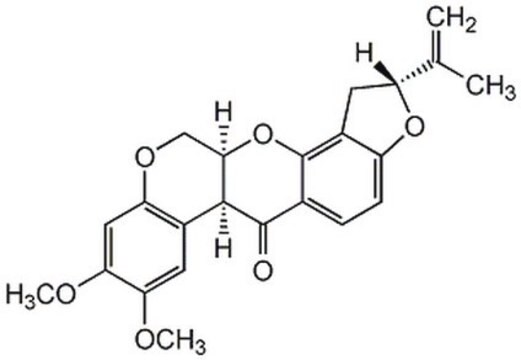
COc1cc2OCC3Oc4c5C[C@@H](Oc5ccc4C(=O)C3c2cc1OC)C(C)=C
InChI
1S/C23H22O6/c1-11(2)16-8-14-15(28-16)6-5-12-22(24)21-13-7-18(25-3)19(26-4)9-17(13)27-10-20(21)29-23(12)14/h5-7,9,16,20-21H,1,8,10H2,2-4H3/t16-,20-,21+/m1/s1
InChI key
JUVIOZPCNVVQFO-HBGVWJBISA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Application
Biochem/physiol Actions
Legal Information
signalword
Danger
Hazard Classifications
Acute Tox. 1 Inhalation - Acute Tox. 2 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
wgk_germany
WGK 3
ppe
dust mask type N95 (US), Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Protocolos
LC/MS/MS Analysis of Pesticide Residues in Pistachios on the Ascentis® Express RP-Amide Column after QuEChERS Extraction
Chromatograms
application for LC-MS, application for SPENuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico