424633
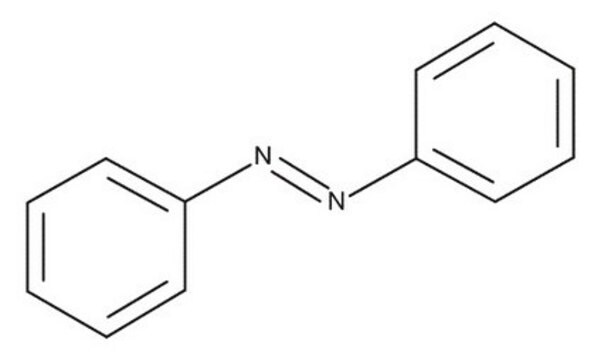
Azobenzene
98%
Sinónimos:
1,2-Diphenyldiazene; Diphenyldiazene
About This Item
Productos recomendados
vapor pressure
1 mmHg ( 104 °C)
Quality Level
assay
98%
form
powder or crystals
autoignition temp.
890 °F
technique(s)
titration: suitable
bp
293 °C (lit.)
mp
65-68 °C (lit.)
density
1.09 g/mL at 25 °C (lit.)
application(s)
diagnostic assay manufacturing
hematology
histology
storage temp.
room temp
SMILES string
c1ccc(cc1)\N=N\c2ccccc2
InChI
1S/C12H10N2/c1-3-7-11(8-4-1)13-14-12-9-5-2-6-10-12/h1-10H/b14-13+
InChI key
DMLAVOWQYNRWNQ-BUHFOSPRSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
General description
Application
As human tissue is translucent to red and near-infrared light but opaque to blue and UV light, Azobenzene is important in medicine and photopharmacology for applications that involve shifting the absorptions of both trans and cis isomers of azobenzene to longer wavelengths.
signalword
Danger
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Carc. 1B - Muta. 2 - STOT RE 2
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 3
flash_point_f
212.0 °F - closed cup
flash_point_c
100.0 °C - closed cup
ppe
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico