8.52000
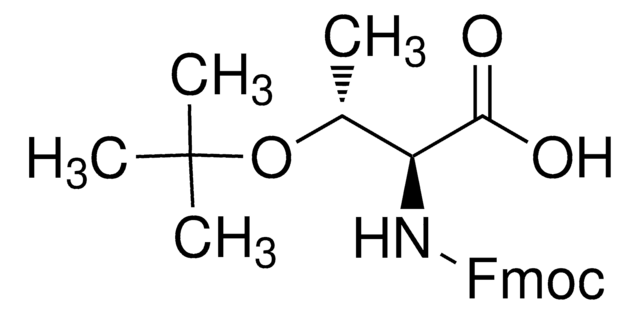
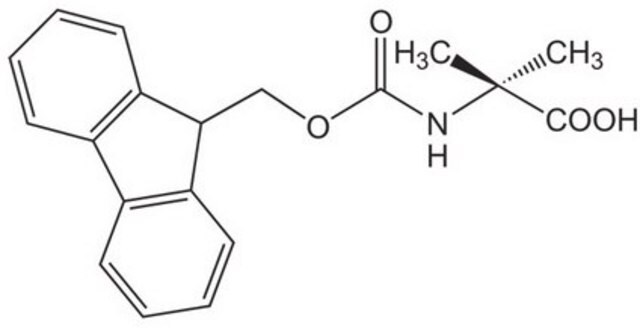
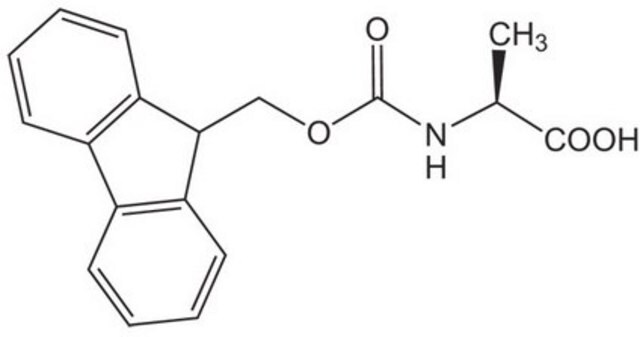
Fmoc-Thr(tBu)-OH
Novabiochem®
Sinónimos:
Fmoc-Thr(tBu)-OH, N-α-Fmoc-O-t.-butyl-L-threonine
About This Item
Productos recomendados
Quality Level
product line
Novabiochem®
assay
≥98% (TLC)
≥98.0% (acidimetric)
≥99.0% (HPLC)
form
powder
reaction suitability
reaction type: Fmoc solid-phase peptide synthesis
manufacturer/tradename
Novabiochem®
mp
125-135 °C
application(s)
peptide synthesis
functional group
hydroxyl
storage temp.
2-30°C
InChI
1S/C23H27NO5/c1-14(29-23(2,3)4)20(21(25)26)24-22(27)28-13-19-17-11-7-5-9-15(17)16-10-6-8-12-18(16)19/h5-12,14,19-20H,13H2,1-4H3,(H,24,27)(H,25,26)/p-1/t14-,20+/m1/s1
InChI key
LZOLWEQBVPVDPR-VLIAUNLRSA-M
General description
Standard building block for introduction of threonine amino-acid residues by Fmoc SPPS
Associated Protocols and Technical Articles
Fmoc-amino acids for Peptide Production
Cleavage and Deprotection Protocols for Fmoc SPPS
Application
- Protocol for Facile Synthesis of Fmoc-N-Me-AA-OH Using 2-CTC Resin as Temporary and Reusable Protecting Group: This study demonstrates the effective use of Fmoc-Thr(tBu)-OH in the synthesis of N-methyl amino acids, which are crucial in developing peptidomimetic drugs (Román et al., 2023).
- Controlled Morphological Changes in Self-Assembled Structures Formed by Fmoc Variants of Threonine and Serine: Explores how Fmoc-Thr(tBu)-OH influences the self-assembly and structural morphology of peptidic materials, relevant in material science (Kshtriya et al., 2021).
- Solid-phase Synthesis of C-terminus Cysteine Peptide Acids: This article includes the use of Fmoc-Thr(tBu)-OH in the synthesis of peptides containing cysteine, which are significant in both drug design and biochemical studies (Mthembu et al., 2022).
- Polymer-Supported Stereoselective Synthesis of Benzoxazinothiadiazepinone 6,6-dioxides: Discusses the use of Fmoc-Thr(tBu)-OH in the stereoselective synthesis of novel organic compounds with potential applications in medicinal chemistry (Králová et al., 2017).
Linkage
Analysis Note
Appearance of substance (visual): powder
Colour index (0,5 M in DMF): ≤ 150 Hazen
Identity (IR): passes test
Enantiomeric purity: ≥ 99.7 % (a/a)
Purity (HPLC): ≥ 99.0 % (a/a)
Fmoc-ß-Ala-OH (HPLC): ≤ 0.1 % (a/a)
Fmoc-ß-Ala-Thr(tBu)-OH (HPLC): ≤ 0.1 % (a/a)
Fmoc-Thr(tBu)-Thr(tBu)-OH (HPLC): ≤ 0.1 % (a/a)
Fmoc-Thr-OH (HPLC): ≤ 0.1 % (a/a)
Assay free amino acid (GC): ≤ 0.2 %
Purity (TLC(011A)): ≥ 98 %
Purity (TLC(0811)): ≥ 98 %
Solubility (25 mmole in 50 ml DMF): clearly soluble
Assay (acidimetric): ≥ 98.0 %
Water (K. F.): ≤ 2.0 %
Ethyl acetate (HS-GC): ≤ 0.5 %
Acetate (IC): ≤ 0.02 %
To see the solvent systems used for TLC of Novabiochem® products please click here.
Legal Information
¿No encuentra el producto adecuado?
Pruebe nuestro Herramienta de selección de productos.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Contenido relacionado
Purer Fmocs Means Purer Peptides
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico