554720
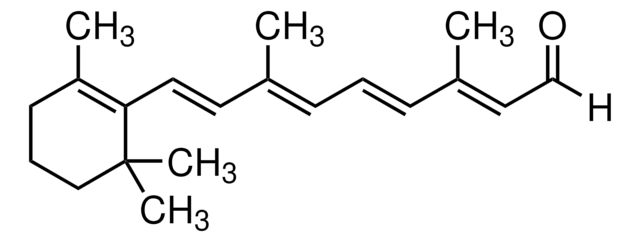
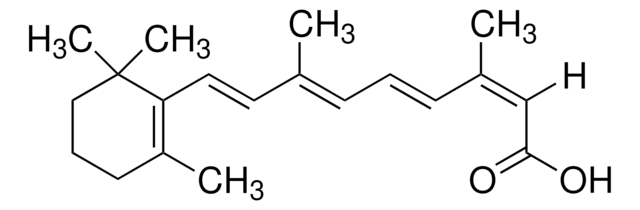
trans-Retinoic Acid
Potent modulator of growth and differentiation. Inhibits melanocyte adhesion, motility, and growth.
Sinónimos:
trans-Retinoic Acid, Tretinoin, ATRA, Vitamin A Acid
About This Item
Productos recomendados
Quality Level
assay
≥95% (by assay)
form
solid
manufacturer/tradename
Calbiochem®
storage condition
OK to freeze
desiccated (hygroscopic)
protect from light
color
yellow
solubility
DMSO: 25 mg/mL
shipped in
ambient
storage temp.
2-8°C
InChI
1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+
InChI key
SHGAZHPCJJPHSC-YCNIQYBTSA-N
Categorías relacionadas
General description
Application
- All-trans-retinoic acid modulates glycolysis via H19 and telomerase: the role of mir-let-7a in estrogen receptor-positive breast cancer cells.: This research reveals how all-trans-retinoic acid influences glycolysis in breast cancer cells by modulating H19 and telomerase, demonstrating its potential in breast cancer therapy (El Habre et al., 2024).
- Retinoic acid tiers mitochondrial metabolism to Sertoli Cell-Mediated efferocytosis via a non-RAR-dependent mechanism.: The study explores the role of retinoic acid in linking mitochondrial metabolism to efferocytosis in Sertoli cells, providing insights into its non-RAR-dependent mechanisms and potential applications in reproductive biology (Wu et al., 2024).
- Combined treatment of All-trans retinoic acid with Tamoxifen suppresses ovarian cancer.: This article discusses the synergistic effects of combining all-trans-retinoic acid with tamoxifen in suppressing ovarian cancer, highlighting a promising therapeutic strategy for ovarian cancer patients (Xu et al., 2024).
Packaging
Warning
Reconstitution
Other Notes
Clagett-Dame, M., et al. 1993. Arch. Biochem. Biophys.300, 684.
Labbaye, C., et al. 1993. Blood81, 475.
Sakashita, A., et al. 1993. Blood81, 1009.
Situ, R., et al. 1993. Dermatology186, 38.
Tini, M., et al. 1993. Genes Develop.7, 295.
Leid, M., et al. 1992. Trends Biochem. Sci.17, 427.
Sharpe, C.R. 1991. Neuron7, 239.
Thaller, C. and Eichele, G. 1990. Nature345, 815.
Legal Information
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Repr. 1B - Skin Irrit. 2
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 2
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico