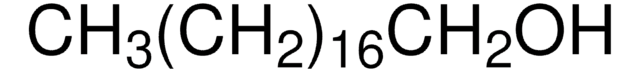1.46481
Buffered NaCl-Peptone Solution with TLH
ready-to-use, bottle volume 1000 mL , filling volume
Sinónimos:
Buffered Saline Peptone Water with neutralizer, Buffered Sodium Chloride Peptone Solution with 0.3% Lecithin, 3% Tween® and 0.1% Histidin
About This Item
Productos recomendados
agency
EP 2.6.13
Quality Level
description
For the microbiological examination of non-sterile pharmaceuticals
sterility
sterile; autoclaved
form
liquid
shelf life
12 mo.
feature
closure type screw cap with septum
ready-to-use
packaging
bottle of
pkg of (1 L in 1 L bottle with blue screw cap and 3 loci (6 bottles per box))
technique(s)
direct inoculation: suitable
sterile filtration: suitable
bottle capacity
1000 mL
bottle volume
1000 mL , filling volume
pH
7.0
application(s)
bioburden testing
cosmetics
food and beverages
pharmaceutical
components
lecithin
histidine
tween
storage temp.
2-25°C
suitability
nonselective for
General description
Application
Features and Benefits
Legal Information
Storage Class
10 - Combustible liquids
wgk_germany
WGK 1
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico