800100O
Avanti
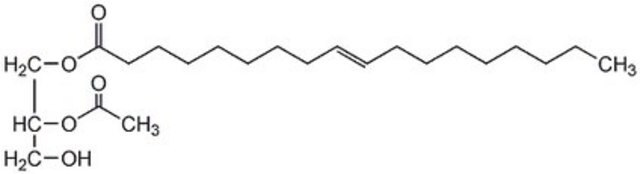
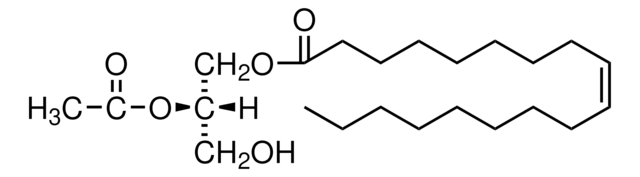
18:1-2:0 DG
1-oleoyl-2-acetyl-sn-glycerol, neat oil
Sinónimos:
1--(9Z-octadecenoyl)-2-acetoyl-sn-glycerol; DG(18:1(9Z)/2:0/0:0); OAG
About This Item
Productos recomendados
assay
>99% (TLC)
form
liquid
packaging
pkg of 1 × 10 mg (with screw cap (800100O-10mg))
pkg of 1 × 25 mg (with screw cap (800100O-25mg))
manufacturer/tradename
Avanti Research™ - A Croda Brand 800100O
lipid type
neutral glycerides
neutral lipids
shipped in
dry ice
storage temp.
−20°C
InChI
1S/C23H42O5/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-23(26)27-20-22(19-24)28-21(2)25/h10-11,22,24H,3-9,12-20H2,1-2H3/b11-10-/t22-/m0/s1
InChI key
PWTCCMJTPHCGMS-YRBAHSOBSA-N
General description
Application
- in the induction of superoxide generation in neutrophils
- as a substrate for platelet-activating factor acetyl hydrolases
- as a protein kinase C (PKC) activator in adrenal glands
Biochem/physiol Actions
Packaging
Storage and Stability
Other Notes
Legal Information
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico