V3700
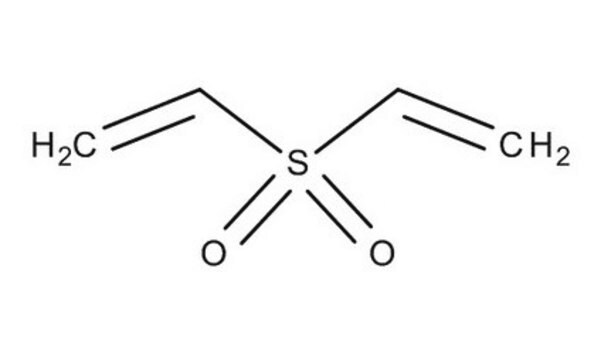
Divinyl sulfone
contains hydroquinone as inhibitor, ≥96%
Sinónimos:
Vinyl sulfone
About This Item
Productos recomendados
Quality Level
assay
≥96%
contains
hydroquinone as inhibitor
refractive index
n20/D 1.476 (lit.)
bp
234 °C (lit.)
mp
−26 °C (lit.)
density
1.177 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
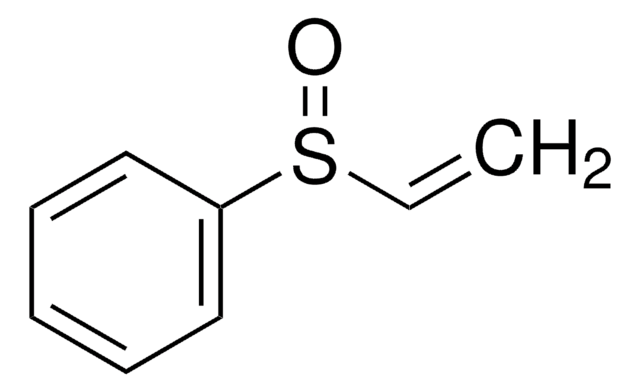
C=CS(=O)(=O)C=C
InChI
1S/C4H6O2S/c1-3-7(5,6)4-2/h3-4H,1-2H2
InChI key
AFOSIXZFDONLBT-UHFFFAOYSA-N
Gene Information
human ... LOC129293(129293)
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
General description
Application
- A cross-linking agent to synthesize divinyl sulfone-crosslinked hyaluronic acid hydrogels for specific biomedical applications, such as tissue engineering or drug delivery.
- A cross-linking agent to develop the conducting polymer film with MXene layers. This crosslinking can enhance the mechanical properties and stability of the composite film.
DVS and its mono and di-substituted derivatives are useful starting materials in the preparation of thiomorpholine 1,1-dioxides and other synthetically important macro- and the heterocycles.
DVS may be used to shrink proofing cotton by crosslinking it with cellulose.
signalword
Danger
Hazard Classifications
Acute Tox. 1 Dermal - Acute Tox. 2 Oral - Aquatic Chronic 3 - Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
wgk_germany
WGK 3
flash_point_f
215.6 °F - closed cup
flash_point_c
102 °C - closed cup
ppe
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico