T71803
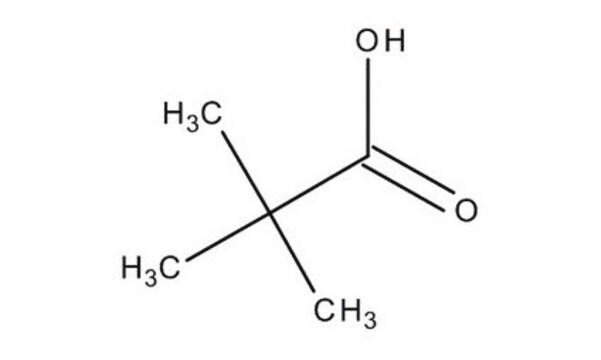
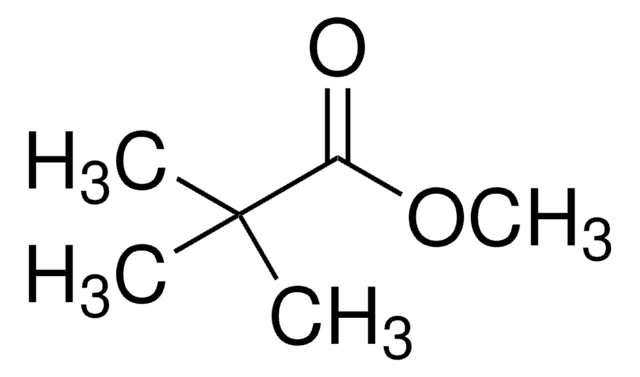
Pivalic acid
99%
Sinónimos:
2,2-Dimethylpropionic acid, Trimethylacetic acid
About This Item
Productos recomendados
vapor density
3.6 (vs air)
Quality Level
vapor pressure
9.75 mmHg ( 60 °C)
assay
99%
reaction suitability
reaction type: C-H Activation
bp
163-164 °C (lit.)
mp
32-35 °C (lit.)
density
0.889 g/mL at 25 °C (lit.)
functional group
carboxylic acid
SMILES string
OC(C(C)(C)C)=O
InChI
1S/C5H10O2/c1-5(2,3)4(6)7/h1-3H3,(H,6,7)
InChI key
IUGYQRQAERSCNH-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
General description
Application
- As a co-catalyst with palladium for the arylation of unactivated arenes and N-heterocycles.
- As an additive to facilitate the carbonylative suzuki reactions to synthesize biaryl ketones from aryl iodides and arylboronic acids by using palladium nanoparticles as catalyst.
- In the cyclization reaction of benzamides with alkynes to synthesize isoquinolones in the presence of 8-aminoquinoline ligand and cobalt catalyst.
Caution
related product
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2
Storage Class
11 - Combustible Solids
wgk_germany
WGK 1
flash_point_f
147.2 °F - closed cup
flash_point_c
64 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Global Trade Item Number
| Número de referencia del producto (SKU) | GTIN |
|---|---|
| T71803-5ML | 4061837372360 |
| T71803-500ML | 4061837372353 |
| T71803-100ML | 4061837372346 |
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico