D74002
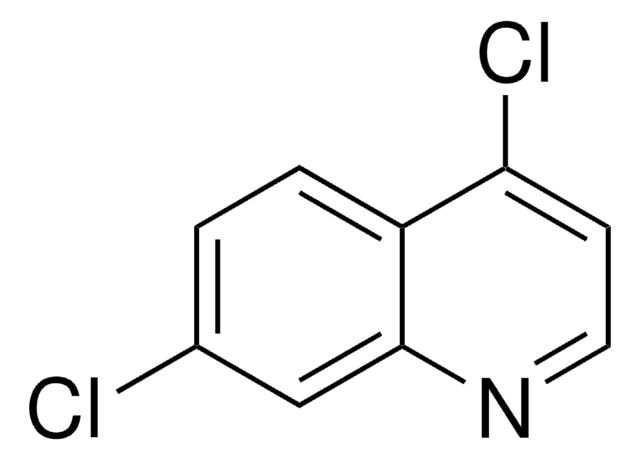
4,7-Dichloroquinoline
97%
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C9H5Cl2N
Número de CAS:
Peso molecular:
198.05
Beilstein/REAXYS Number:
125359
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
assay
97%
form
powder
mp
81-83 °C (lit.)
SMILES string
Clc1ccc2c(Cl)ccnc2c1
InChI
1S/C9H5Cl2N/c10-6-1-2-7-8(11)3-4-12-9(7)5-6/h1-5H
InChI key
HXEWMTXDBOQQKO-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
signalword
Warning
hcodes
Hazard Classifications
Aquatic Chronic 2 - Eye Irrit. 2 - Skin Irrit. 2 - Skin Sens. 1
Storage Class
13 - Non Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Elaine S Coimbra et al.
Chemical biology & drug design, 75(6), 628-631 (2010-03-27)
We report herein the condensation of 4,7-dichloroquinoline (1) with tryptamine (2) and D-tryptophan methyl ester (3). Hydrolysis of the methyl ester adduct (5) yielded the free acid (6). The compounds were evaluated in vitro for activity against four different species
J T Mague et al.
Acta crystallographica. Section C, Crystal structure communications, 51 ( Pt 7), 1423-1425 (1995-07-15)
The title compound C14H12C12N2O, has been shown to have an E configuration about the double bond in the propenal moiety. Significant delocalization of the lone pair on the N atom of the dimethylamino group into the pi system of this
Allergic contact dermatitis from 4,7-dichloroquinoline.
F C Pickering et al.
Contact dermatitis, 8(4), 269-270 (1982-07-01)
Mostafa M Ghorab et al.
Acta pharmaceutica (Zagreb, Croatia), 64(3), 285-297 (2014-10-10)
Novel nineteen compounds based on a 4-aminoquinoline scaffold were designed and synthesized as potential antiproliferative agents. The new compounds were N-substituted at the 4-position by aryl or heteroaryl (1-9), quinolin- 3-yl (10), 2-methylquinolin-3-yl (11), thiazol-2-yl (12), and dapsone moieties (13
Howaida I Abd-Alla et al.
Natural product research, 23(11), 1035-1049 (2009-06-13)
The chemical constituents and biological activities of leaves and roots of Aloe hijazensis, collected in Saudi Arabia, are reported here for the first time. Twenty-two compounds were obtained, among them eight hydroxyquinones: aloe-emodin (1), emodin (2), chrysophanol (3), aloesaponarin II
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico