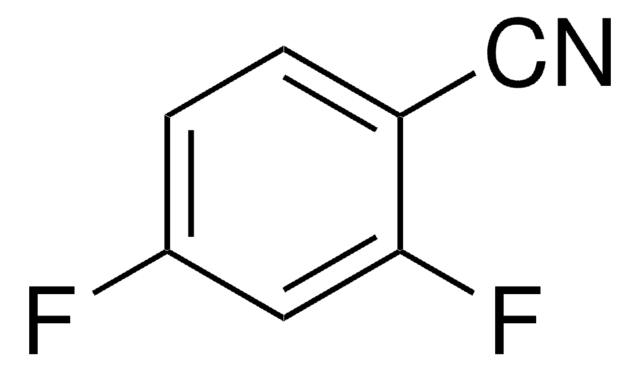
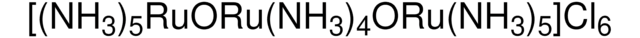D57558
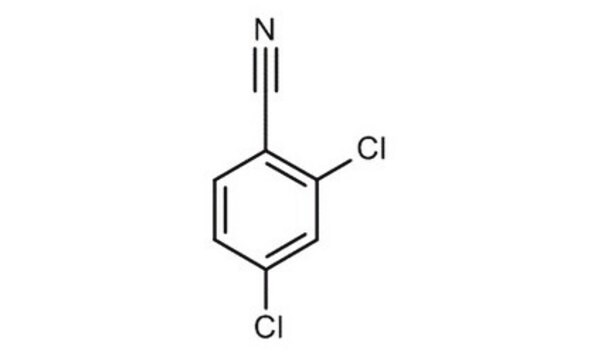
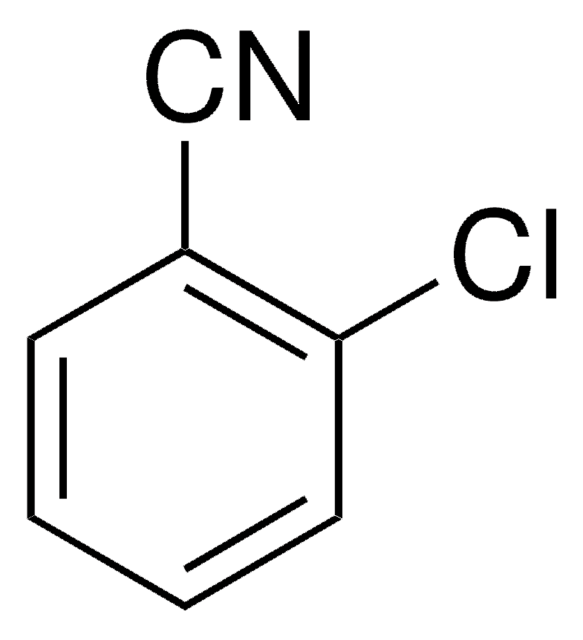
2,6-Dichlorobenzonitrile
97%
Sinónimos:
Dichlobenil
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
Cl2C6H3CN
Número de CAS:
Peso molecular:
172.01
Beilstein/REAXYS Number:
1909167
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
assay
97%
form
powder
mp
143-146 °C (lit.)
SMILES string
Clc1cccc(Cl)c1C#N
InChI
1S/C7H3Cl2N/c8-6-2-1-3-7(9)5(6)4-10/h1-3H
InChI key
YOYAIZYFCNQIRF-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Application
2,6-Dichlorobenzonitrile can be used as a starting material to synthesize:
- 2,6-Dichlorobenzaldehyde using lithium N, N′-dimethylethylenediaminoaluminum hydride as a reducing agent.
- 5-(2,6-Dichlorophenyl)-2H-tetrazole via gold-catalyzed nucleophilic (3 + 2) cycloaddition reaction with sodium azide.
- 2,6-Dichlorobenzamide via hydrolysis using potassium tert-butoxide as a catalyst.
- Chloro-aminoindazole by reacting with hydrazine monohydrate.
- 2,6-Dichlorobenzenecarboselenoamide by treating with Woollins′ reagent.
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Dermal - Aquatic Chronic 2
Storage Class
11 - Combustible Solids
wgk_germany
WGK 2
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Synthesis of primary arylselenoamides by reaction of aryl nitriles with Woollins' reagent
Hua Guoxiong, et al.
Organic Letters, 8(23), 5251-5254 (2006)
Valeria Franceschini et al.
Stem cells (Dayton, Ohio), 27(4), 825-835 (2009-04-08)
The herbicide dichlobenil selectively causes necrosis of the dorsomedial part of olfactory neuroepithelium (NE) with permanent damage to the underlying mucosa, whereas the lateral part of the olfactory region and the nasal respiratory mucosa remain undamaged. We investigated here whether
L Peng et al.
Plant biology (Stuttgart, Germany), 15(2), 405-414 (2012-07-05)
Cellulose is the major component of plant cell walls and is an important source of industrial raw material. Although cellulose biosynthesis is one of the most important biochemical processes in plant biology, the regulatory mechanisms of cellulose synthesis are still
Fang Xie et al.
Toxicology and applied pharmacology, 272(3), 598-607 (2013-08-08)
We explored the mechanisms underlying the differential effects of two olfactory toxicants, the herbicide 2,6-dichlorobenzonitrile (DCBN) and the anti-thyroid drug methimazole (MMZ), on olfactory receptor neuron (ORN) regeneration in mouse olfactory epithelium (OE). DCBN, but not MMZ, induced inflammation-like pathological
Guang-Cai Chen et al.
Journal of hazardous materials, 188(1-3), 156-163 (2011-02-18)
The effect of lead on the adsorption of diuron and dichlobenil on multiwalled carbon nanotubes (MWCNTs) was investigated to explore the possible application of MWCNTs for removal of both herbicides from contaminated water. The adsorption of diuron and dichlobenil on
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico