779776
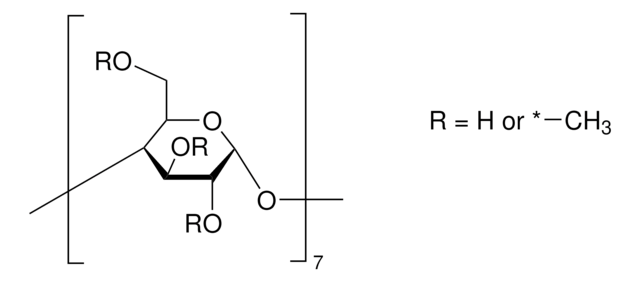
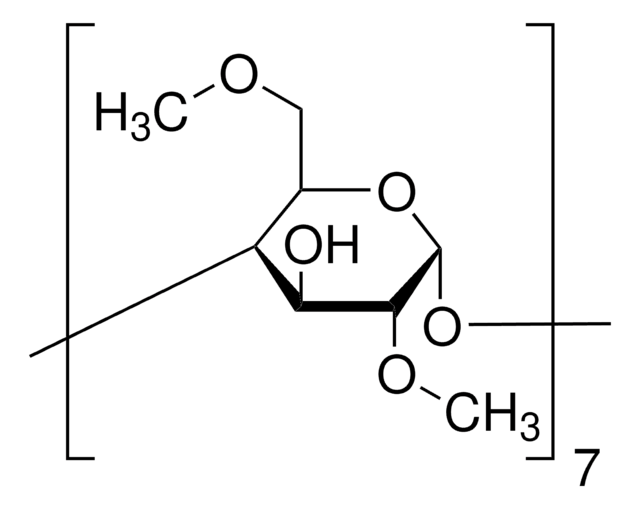
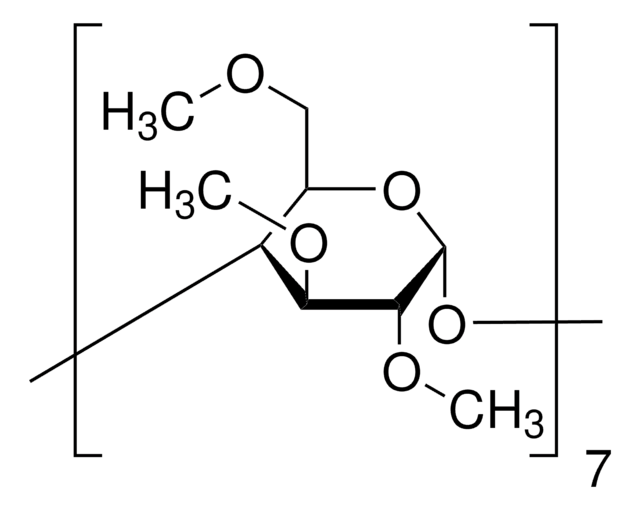
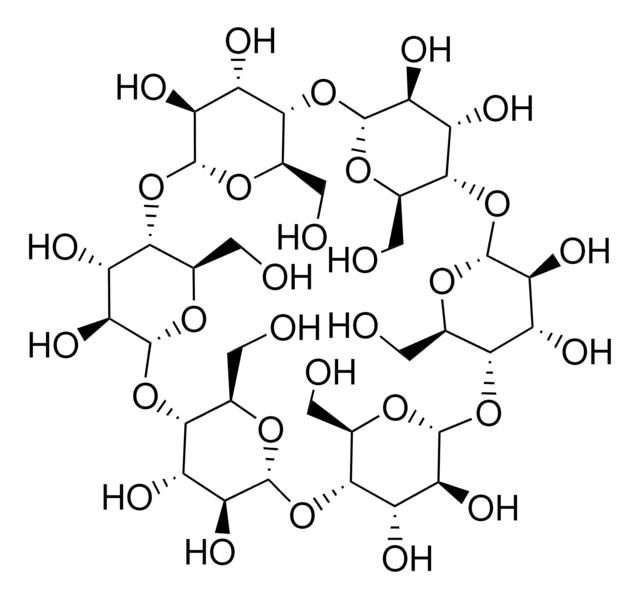
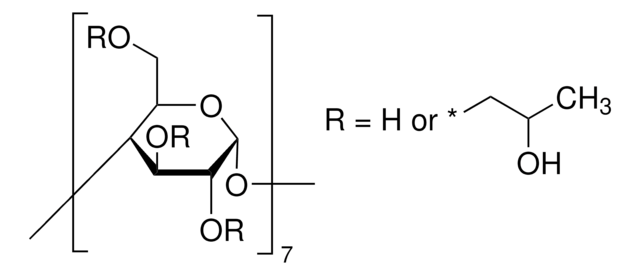
Metil-β-ciclodextrina
produced by Wacker Chemie AG, Burghausen, Germany, ≥95.0% cyclodextrin basis (calculated)
Sinónimos:
Cavasol® W7 M, Methyl-beta-cyclodextrin
About This Item
Productos recomendados
grade
produced by Wacker Chemie AG, Burghausen, Germany
Quality Level
assay
≥95.0% cyclodextrin basis (calculated)
form
powder
extent of labeling
1.6-1.9 molar substitution
impurities
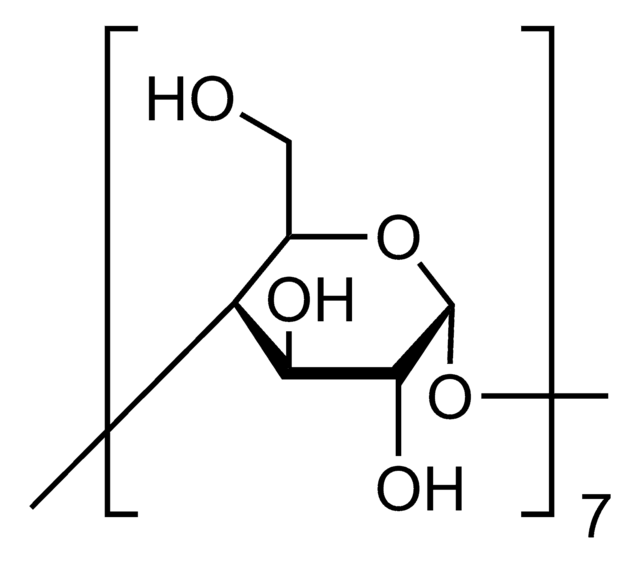
≤0.2% unsubstituted cyclodextrin
≤0.2% volatile organics
≤2% chloride
loss
≤7.0% loss on drying
mp
180-182 °C (lit.)
InChI
1S/C56H98O35/c1-64-15-22-36-29(57)43(71-8)50(78-22)86-37-23(16-65-2)80-52(45(73-10)30(37)58)88-39-25(18-67-4)82-54(47(75-12)32(39)60)90-41-27(20-69-6)84-56(49(77-14)34(41)62)91-42-28(21-70-7)83-55(48(76-13)35(42)63)89-40-26(19-68-5)81-53(46(74-11)33(40)61)87-38-24(17-66-3)79-51(85-36)44(72-9)31(38)59/h22-63H,15-21H2,1-14H3
InChI key
QGKBSGBYSPTPKJ-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
Application
Other Notes
Legal Information
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
368.6 °F
flash_point_c
187 °C
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico