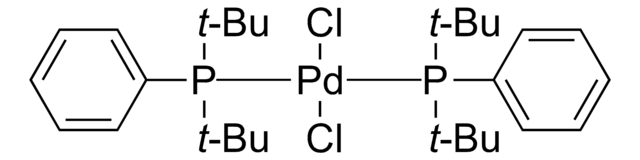
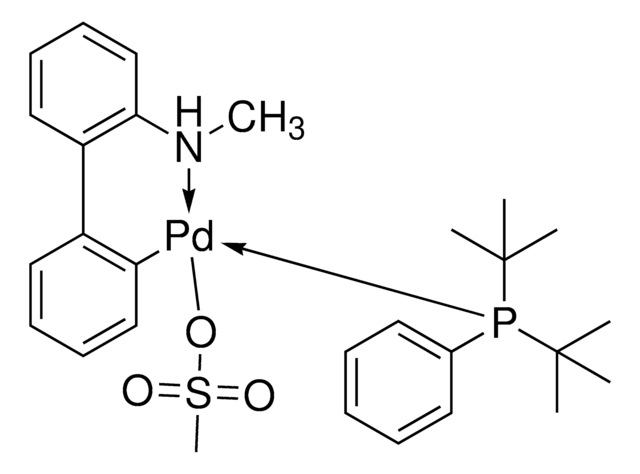
756482
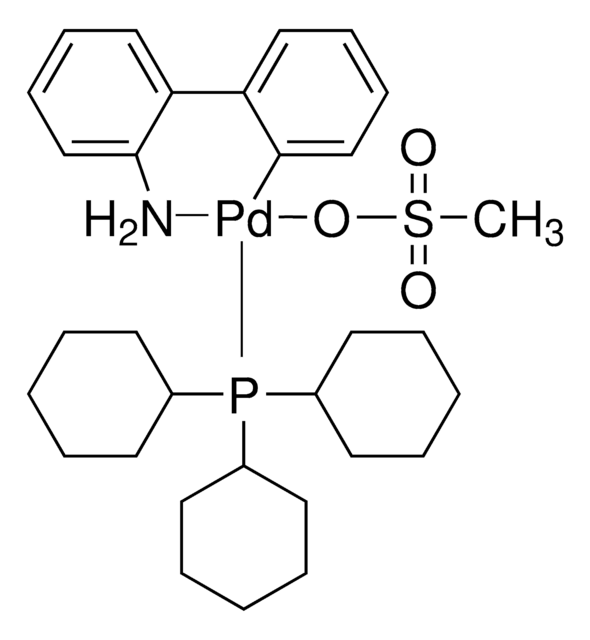
P(t-Bu)3 Pd G2
Sinónimos:
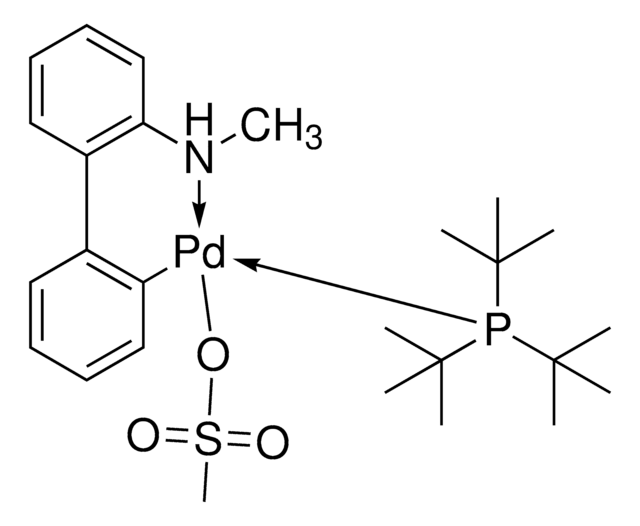
Chloro[(tri-tert-butylphosphine)-2-(2-aminobiphenyl)] palladium(II)
About This Item
Productos recomendados
form
solid
Quality Level
feature
generation 2
reaction suitability
core: palladium
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
reaction type: Cross Couplings
mp
167-170 °C (decomposition)
functional group
phosphine
SMILES string
NC1=C(C2=CC=CC=C2[Pd]Cl)C=CC=C1.CC(C)(C)P(C(C)(C)C)C(C)(C)C
InChI
1S/C12H10N.C12H27P.ClH.Pd/c13-12-9-5-4-8-11(12)10-6-2-1-3-7-10;1-10(2,3)13(11(4,5)6)12(7,8)9;;/h1-6,8-9H,13H2;1-9H3;1H;/q;;;+1/p-1
InChI key
ZVSLIOFJVMRWHJ-UHFFFAOYSA-M
General description
Application
- Synthesis of sterically hindered biaryls (tetra-ortho-substituted), via cross-coupling reactions of aryl chlorides.
- Stille cross-couplings reactions of aryl chloride.
- Synthesis of chloropeptin I, via Stille cross-coupling reaction.
- Heck reaction.
- Negishi cross-coupling reactions.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
All of the preformed catalysts used in the kit are air and moisture stable complexes in their commercially available form. Once activated by base under the reaction conditions they become sensitive to air. To best enable scale-up success, the use of standard Schlenk technique is recommended.
All contents in the foil bag are weighed, plated, packed, and sealed in a glove box under nitrogen.
Contenido relacionado
The Buchwald group has developed a series of highly active and versatile palladium precatalysts and biarylphosphine ligands used in cross-coupling reactions for the formation of C-C, C–N, C–O, C–F, C–CF3, and C–S bonds. The ligands are electron-rich, and highly tunable to provide catalyst systems with a diverse scope, high stability and reactivity. Furthermore, the new series of precatalysts are air-, moisture and thermally-stable and display good solubility in common organic solvents. The use of precatalysts ensures the efficient generation of the active catalytic species and allows one to accurately adjust the ligand:palladium ratio. The ligands, precatalysts and methodology developed in the Buchwald group are user friendly and have rendered previously difficult cross couplings reactions, much easier to achieve.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico
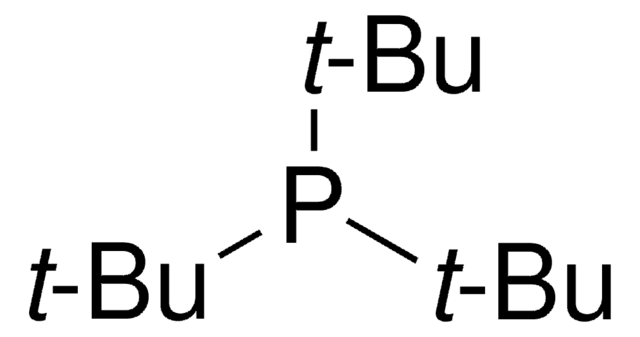
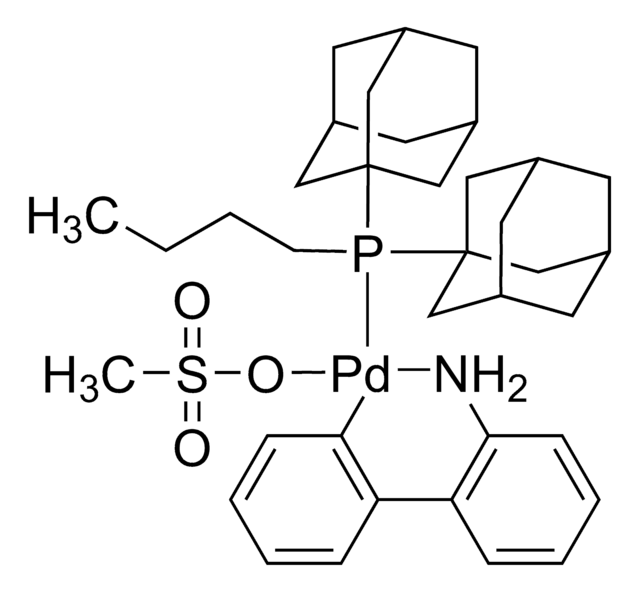
![Mesyl[(tri-t-butylphosphine)-2-(2-aminobiphenyl)]palladium(II)](/deepweb/assets/sigmaaldrich/product/structures/358/298/6539c19e-808c-4cd1-b9e8-19c6928f2384/640/6539c19e-808c-4cd1-b9e8-19c6928f2384.png)