697230
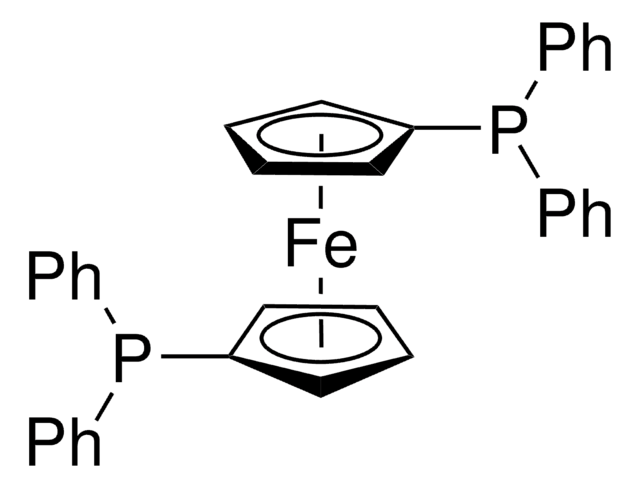
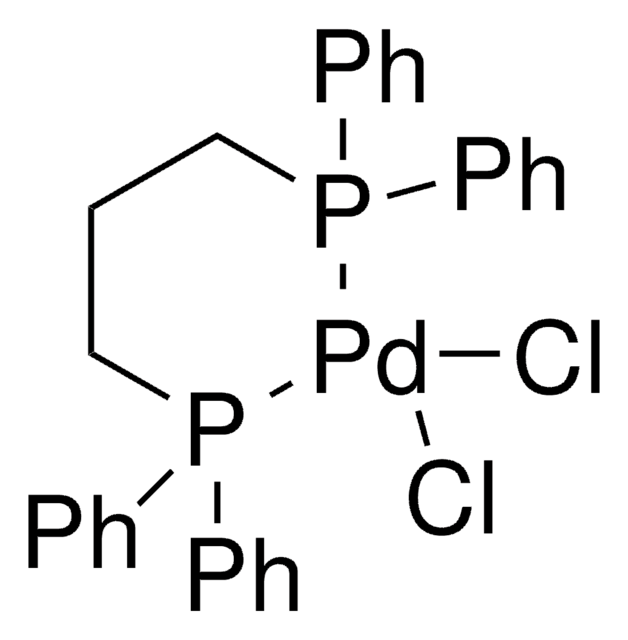
[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)
Sinónimos:
Pd(dppf)Cl2
About This Item
Productos recomendados
form
powder
Quality Level
reaction suitability
core: palladium
reaction type: Cross Couplings
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
reaction type: Buchwald-Hartwig Cross Coupling Reaction
mp
266-283 °C (lit.)
SMILES string
[Fe].Cl[Pd]Cl.[CH]1[CH][CH][C]([CH]1)P(c2ccccc2)c3ccccc3.[CH]4[CH][CH][C]([CH]4)P(c5ccccc5)c6ccccc6
InChI
1S/2C17H14P.2ClH.Fe.Pd/c2*1-3-9-15(10-4-1)18(17-13-7-8-14-17)16-11-5-2-6-12-16;;;;/h2*1-14H;2*1H;;/q;;;;;+2/p-2
InChI key
JCWIWBWXCVGEAN-UHFFFAOYSA-L
¿Está buscando productos similares? Visita Guía de comparación de productos
Application
- Cross-coupling of sec-alkyl and n-alkyl Grignard reagents with high yield and selectivity.
- Suzuki coupling of aryl boronic esters with [11C]methyl iodide to form functionalized [11C]toluene derivatives.
- Kumada cross-coupling of 1,3,5-tribromobenzene with Grignard reagents to form star-shaped oligothiophenes.
Related product
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Tandem hydroboration Suzuki Coupling both intermolecular and intramolecular gave diverse alkyl substituted products dppf
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico![[1,1′-bis(difenilfosfino)ferroceno]dicloropaladio(II), complejo con diclorometano](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)




![[Pd(OAc)2]3 reagent grade, 98%](/deepweb/assets/sigmaaldrich/product/structures/508/249/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f/640/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f.png)