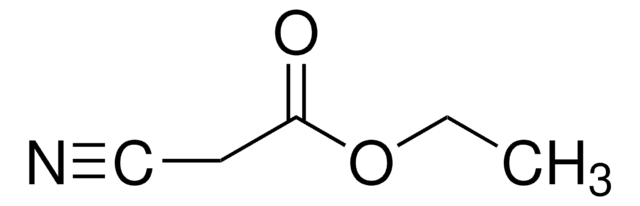
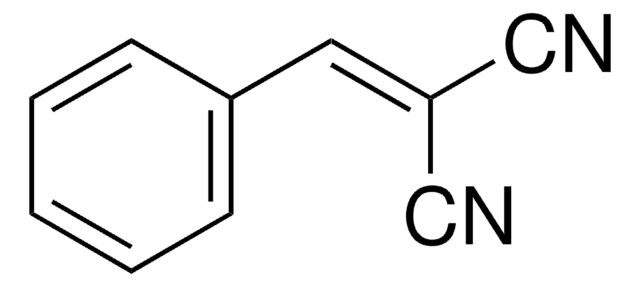
690414
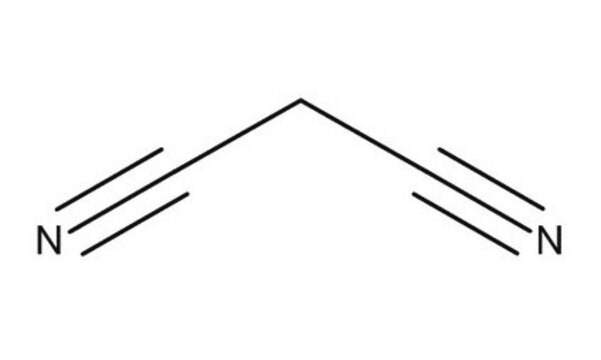
Malononitrile
Arxada quality, ≥99.0% (calculated, GC, KF)
Sinónimos:
Dicyanomethane
About This Item
Productos recomendados
Quality Level
assay
≥99.0% (calculated, GC, KF)
form
liquid
quality
Arxada quality
manufacturer/tradename
Arxada AG
impurities
≤0.10% water
≤0.50% (E)-2-butenedinitrile
≤0.50% (Z)-2-butenedinitrile
≤0.50% butanedinitrile
bp
220 °C (lit.)
mp
30-32 °C (lit.)
density
1.049 g/mL at 25 °C (lit.)
functional group
nitrile
storage temp.
2-8°C
SMILES string
N#CCC#N
InChI
1S/C3H2N2/c4-2-1-3-5/h1H2
InChI key
CUONGYYJJVDODC-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Application
Some of the reactions where malononitrile is used as a reactant are:
- Synthesis of 2-pyran-4-ylidene-malononitrile (PM) based red light emitting polymers.
- Synthesis of polysubstituted dihydropyridines.
- Synthesis of various chromene derivatives upon treating with salicylic aldehydes.
- Synthesis of triselenium dicyanide by treating it with selenium dioxide.
signalword
Danger
Hazard Classifications
Acute Tox. 2 Oral - Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Irrit. 2 - Skin Sens. 1
Storage Class
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
wgk_germany
WGK 3
flash_point_f
186.8 °F - closed cup
flash_point_c
86 °C - closed cup
ppe
dust mask type N95 (US), Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico
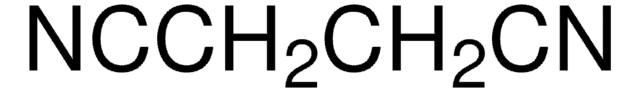
![2-[Bis(methylthio)methylene]malononitrile 97%](/deepweb/assets/sigmaaldrich/product/structures/144/342/6a420594-3bce-4984-a8b7-5bf2a92d6a97/640/6a420594-3bce-4984-a8b7-5bf2a92d6a97.png)