391573
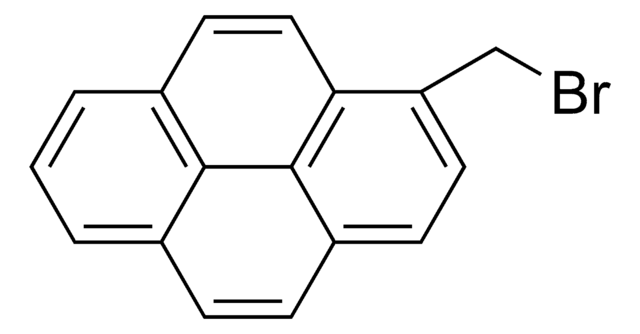
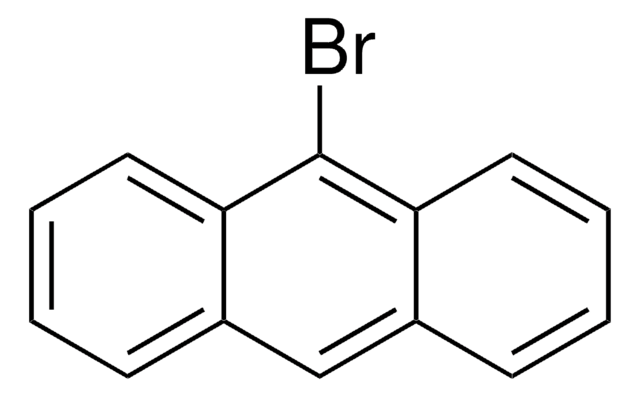
1-Bromopyrene
96%
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C16H9Br
Número de CAS:
Peso molecular:
281.15
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
96%
form
powder
mp
102-105 °C (lit.)
SMILES string
Brc1ccc2ccc3cccc4ccc1c2c34
InChI
1S/C16H9Br/c17-14-9-7-12-5-4-10-2-1-3-11-6-8-13(14)16(12)15(10)11/h1-9H
InChI key
HYGLETVERPVXOS-UHFFFAOYSA-N
General description
1-Bromopyrene, a polycyclic aromatic hydrocarbon (PAH), is a mono bromo substituted pyrene derivative. Its synthesis has been reported. Its gas phase UV-absorption spectra in the UV wavelength range at elevated temperature has been studied. Electron affinitiy (EA) of 1-bromopyrene has been investigated using electron attachment desorption chemical ionization mass spectrometry (DCI-MS) and triple quadrupole tandem mass spectrometry. It participates in the synthesis of novel ruthenium (II) bipyridine or terpyridine complexes bearing pyrene moiety. The reaction of 1-bromopyrene cation radical with water in acetonitrile has been analyzed using the electron transfer stopped-flow (ETSF) method.
Application
1-Bromopyrene is suitable reagent used in the comparative study of effect of substituents of some pyrene derivatives in inducing phototoxicity, DNA damage and repair in human skin keratinocytes and light-induced lipid peroxidation in methanol. It is suitable reagent used in the study to investigate the UV photon-assisted thermal decomposition of PAHs at elevated temperature.
1-Bromopyrene may be used as a standard to compare its spectral properties with that of pyrene based fluorescence probe. It may be used to study the effects of the addition of halogen hetero-atoms on the vapor pressures and thermodynamics of polycyclic aromatic hydrocarbons.
It may be used in the synthesis of the following:
1-Bromopyrene may be used as a standard to compare its spectral properties with that of pyrene based fluorescence probe. It may be used to study the effects of the addition of halogen hetero-atoms on the vapor pressures and thermodynamics of polycyclic aromatic hydrocarbons.
It may be used in the synthesis of the following:
- 2-methyl-4-pyren-1-yl-but-3-yn-2-ol
- 1-ethynylpyrene
- silsesquioxane (SSQ) based hybrid
- ruthenium nanoparticles functionalized with pyrene moiety
- mono- and di-pyrenyl perfluoroalkanes
- oligo(1-bromopyrene)(OBrP) films
- dinitropyrene-derived DNA adduct
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Electron affinities of polycyclic aromatic hydrocarbons determined by the kinetic method.
Chen G and Cooks RG.
Journal of Mass Spectrometry : Jms, 30(8), 1167-1173 (1995)
Pyrene-functionalized ruthenium nanoparticles: novel fluorescence characteristics from intraparticle extended conjugation.
Chen W, et al.
The Journal of Physical Chemistry C, 113(39), 16988-16995 (2009)
Novel fluorescence probe based on pyrene and piperazine; spectral properties in solution and in polymer matrices.
Hrdlovic P et al.
Journal of Photochemistry and Photobiology A: Chemistry, 163(1), 289-296 (2004)
Tracie Perkins Fullove et al.
Toxicology research, 2(3), 193-199 (2014-06-06)
Polycyclic aromatic hydrocarbons (PAHs), a class of mutagenic environmental contaminants, insert toxicity through both metabolic activation and light irradiation. Pyrene, one of the most widely studied PAHs, along with its mono-substituted derivatives, 1-amino, 1-bromo, 1-hydroxy, and 1-nitropyrene, were chosen to
UV photon-assisted incineration of polycyclic aromatic hydrocarbons at elevated temperatures between 150 and 800?C.
Thony A and Rossi MJ.
Journal of Photochemistry and Photobiology A: Chemistry, 109(3), 267-280 (1997)
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico