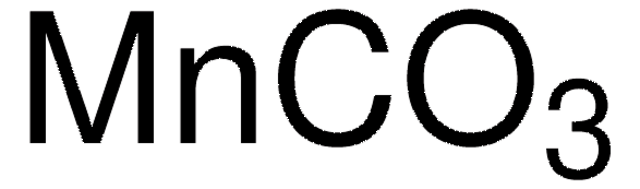377449
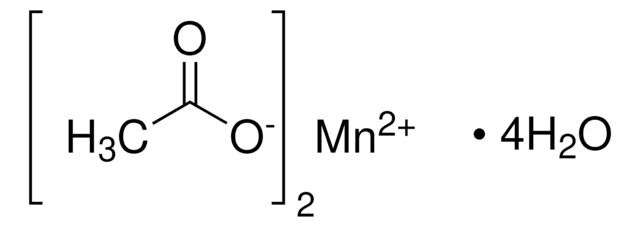
Manganese(II) carbonate
≥99.9% trace metals basis
Sinónimos:
Manganese carbonate, Manganese(2+) carbonate, Manganous carbonate
About This Item
Productos recomendados
Quality Level
assay
≥99.9% trace metals basis
form
powder
impurities
≤1,000.0 ppm Trace Metal Analysis
mp
>200 °C (lit.)
solubility
dilute aqueous acid: slightly soluble(lit.)
density
3.12 g/mL at 25 °C (lit.)
SMILES string
[Mn++].[O-]C([O-])=O
InChI
1S/CH2O3.Mn/c2-1(3)4;/h(H2,2,3,4);/q;+2/p-2
InChI key
XMWCXZJXESXBBY-UHFFFAOYSA-L
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Application
- A primary electrode material in asymmetric supercapacitors for improving the charge storage capacity and overall performance of the supercapacitors.
- A precursor for synthesizing manganese oxide, which is used as a component in various electrochemical applications, including batteries and supercapacitors.
- A precursor material in the synthesis of oxygen vacancy-rich nitrogen-doped manganese carbonate (MnCO2@N) microspheres. These microspheres are then employed as cathode materials in aqueous zinc-ion batteries (ZIBs) to enhance their electrochemical performance.
- A potential electrocatalyst for the oxygen evolution reaction (OER) in water splitting applications.
Storage Class
13 - Non Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Recently, layer-by-layer (LbL) assembly has emerged as a versatile, gentle and, simple method for immobilization of functional molecules in an easily controllable thin film morphology.1,2 In this short review, we introduce recent advances in functional systems fabricated by using the mild, yet adaptable LbL technique.
Thermoelectric Performance of Perovskite-type Oxide Materials
The prevailing strategies for heat and electric-power production that rely on fossil and fission fuels are having a negative impact on the environment and on our living conditions.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico